гђђ2 Stepsгђ Cl2 Lewis Structure Lewis Dot Structure For Chlorineођ

How To Draw The B3 Lewis Dot Structure Youtube A step by step explanation of how to draw the cl2 lewis dot structure (chlorine gas).for the cl2 structure use the periodic table to find the total number of. Molecular geometry of cl 2. cl 2 has a linear electron geometry. this is due to the fact that all diatomic molecules or any molecule with only two atoms will have a linear geometry or shape as these molecules contain two atoms that are connected with a single bond. hybridization in cl 2. each chlorine atom in the cl 2 lewis structure has an sp.
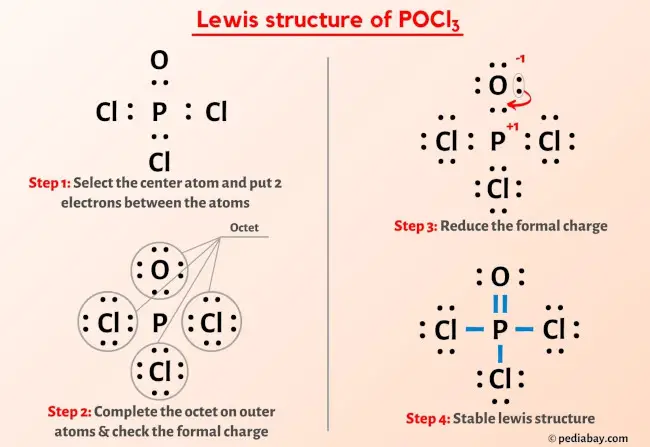
Cl3po Lewis Structure The lewis structure generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules. to use the lewis structure calculator follow these steps: enter the formula of the molecule in the field provided for it. for example, if we want to obtain the lewis structure of the sulfate ion, so4– 2, we must. At room temperature, it is a yellow gas with a pungent odor. it has a high density (3.2 g ml). the molecule is almost neutral (ph=7.4). it is slightly soluble in water. the boiling and melting point of molecular chlorine are 239.11k and 171.6k, respectively. cl 2 is a covalent molecule as the bond is formed by sharing of electrons. Step #1: calculate the total number of valence electrons. here, the given molecule is cl2 (chlorine). in order to draw the lewis structure of cl2, first of all you have to find the total number of valence electrons present in the cl2 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). A step by step explanation of how to draw the cl2 lewis dot structure (diatomic chlorine).note that diatomic chlorine is often called molecular chlorine or j.

Lewis Diagram For Cl2 Step #1: calculate the total number of valence electrons. here, the given molecule is cl2 (chlorine). in order to draw the lewis structure of cl2, first of all you have to find the total number of valence electrons present in the cl2 molecule. (valence electrons are the number of electrons present in the outermost shell of an atom). A step by step explanation of how to draw the cl2 lewis dot structure (diatomic chlorine).note that diatomic chlorine is often called molecular chlorine or j. Follow these simple steps to draw lewis dot structures: draw the atoms on paper and put dots around them to represent valence electrons of the atom. be sure to have the correct number of electrons. if the species is an ion, add or subtract electrons corresponding to the charge of the ion. add an electron for every negative ( ) charge, and. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. the number of dots equals the number of valence electrons in the atom. these dots are arranged to the right and left and above and below the.

Comments are closed.