13 Saturated Liquid Approximation And Getting Properties From

13 Saturated Liquid Approximation And Getting Properties From Saturated liquid specific internal energy is, u1 = 1080.4 kj kg. (3) the specific volume at state 2 will be identical to the specific volume at state 1 since the tank volume and water mass remain unchanged, i.e., v2 = v1 = 1.25*10 3 m3 kg. (4) at p2 = 35 bar (abs), the specific volumes for the saturated liquid and saturated vapor states (table a 3). – the two phase (liquid vapor) dome shaped region is known as the vapor dome. – the lines bordering the vapor dome are known as the saturated liquid and saturated vapor lines. – the point at the top of the dome is known as the critical point, which is at the critical temper ature, t c, and critical pressure, p c, and critical specific.

Ppt Engsc 2333 Thermodynamics Chapter 3 Powerpoint Presentation Pressure, p. saturated liquid line. 6.4 enthalpy the sum of the internal energy u and the pressure multiplied by the volume pv frequently appears in thermo fluid analyses and so is given the special name, enthalpy, h, h ≡ u pv . (104) note that enthalpy is a property since u, p, and v are also properties. on a per cl: unit mass compressed. About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. T he compressed liquid table is presented only for water in the pressure range of 0.5 50 mpa i n this book. when the compressed liquid tables are not available for a specific fluid or in a specific range, the saturated liquid properties at the same temperature may be used as an approximation, i.e., , , , and . Quality is between 0 and 1 0: sat. liquid, 1: sat. vapor. the properties of the saturated liquid are the same whether it exists alone or in a mixture with saturated vapor. the relative amounts of liquid and vapor phases in a saturated mixture are specified by the quality x. a two phase system can be treated as a homogeneous.
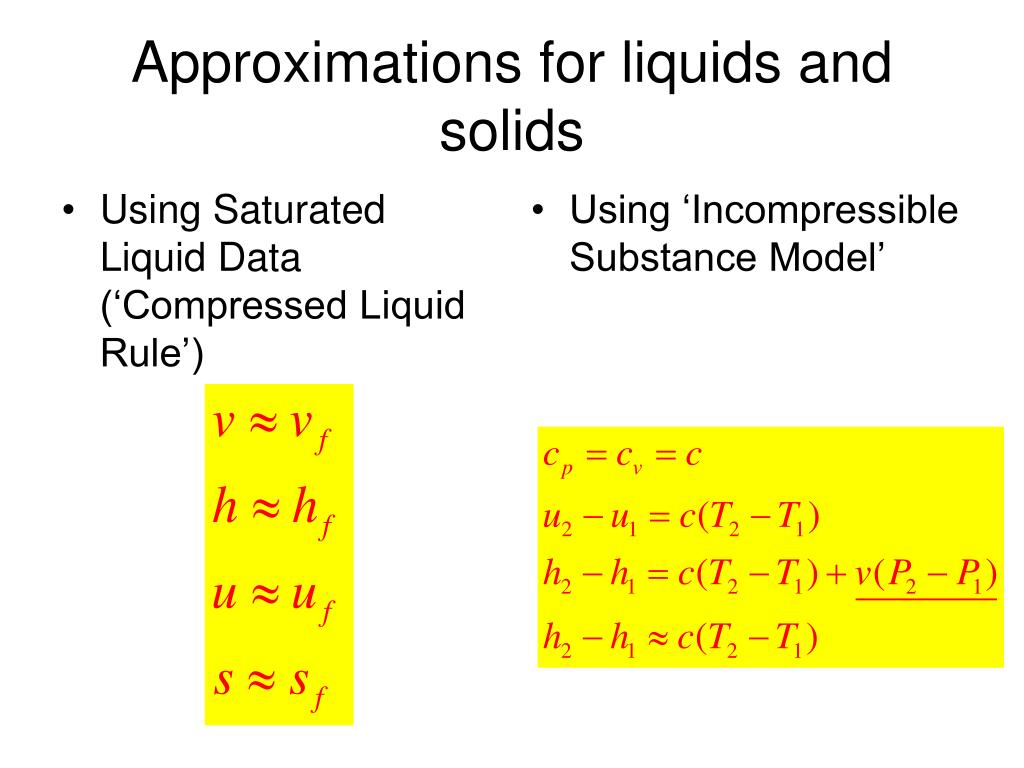
Ppt Chapter 3 Evaluating Properties Powerpoint Presentation Free T he compressed liquid table is presented only for water in the pressure range of 0.5 50 mpa i n this book. when the compressed liquid tables are not available for a specific fluid or in a specific range, the saturated liquid properties at the same temperature may be used as an approximation, i.e., , , , and . Quality is between 0 and 1 0: sat. liquid, 1: sat. vapor. the properties of the saturated liquid are the same whether it exists alone or in a mixture with saturated vapor. the relative amounts of liquid and vapor phases in a saturated mixture are specified by the quality x. a two phase system can be treated as a homogeneous. Saturated fluid properties. if the gas or vapor represented by the point x in the phase for a pure fluid shown in figure 1 is compressed slowly and isothermally, the pressure rises until the vapor becomes saturated and the first drop of liquid appears at conditions corresponding to point 1. if the compression is continued, condensation takes. Process 1 2: the temperature and specific volume will increase from the compressed liquid, or subcooled liquid, state 1, to the saturated liquid state 2. in the compressed liquid region, the properties of the liquid are approximately equal to the properties of the saturated liquid state at the temperature. process 2 3:.

Comments are closed.