Activation Energy In Chemical Kinetics Pre Lab Nyb Chemistry Of Solutions

Activation Energy In Chemical Kinetics Pre Lab Nyb Chemistry Of Activation energy in chemical kineticspre laboratory experimental procedure for the dawson college nyb chemistry of solutions pre university course.the oxida. Mandatory assignments. date. rating. year. ratings. lab report 1 volumetric analysis of an acid solution copy. rate law kinematics ousman djibril (c.s 202 nyb 05) haniff. chemical equilibrium i the equilibrium constant (lab) show 8 more documents.

Collision Theory Arrhenius Equation Activation Energy Chemical The rate of a reaction is the change in concentration of a reactant or a product per unit of time. the goal of this experiment was to determine the rate law, which only takes into account the reactants, and the constant k of the following reaction: 2 i (−aq¿ )s 2 o¿ 2 −¿→ i 2 (aq) 2 so¿ 2 −¿¿¿ ¿. rate = k [i−¿¿]m [s 2 o 82. All the pre lab experimental videos for the chemistry of solutions course (202 nyb 05) created by the dawson college chemistry department are collected in th. Chemistry questions and answers. chemical kinetics i: rate law and activation energy name date pre lab questions 1. the following rate data were collected for the reaction at 100°c 2 no, f2 2 nof no] (m) 0.0480 0.0120 0.0480 [f,] (m) 0.0317 0.0317 0.127 rate (m s) 0.00190 0.000469 0.00757 2 3 determine the reaction order for no and f2 a. b. Experiment #8 activation energy in chemical kinetics department of chemistry and chemical technology chemistry of solutions, 202 nyb 05, section: 04, winter semester 2020 farbod niazi 1934663 partner: arshia tehrani mehr 1934719 dr. rodney s.t. squire performed on may 11, 2020 submitted on may 18, 2020.
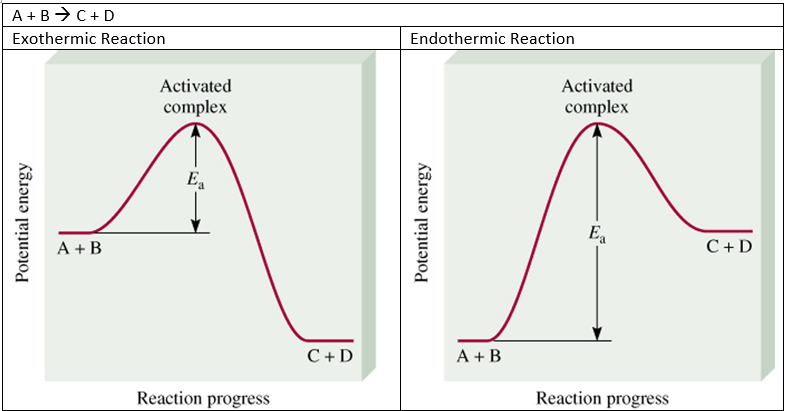
Activation Energy In Chemical Kinetics W3schools Chemistry questions and answers. chemical kinetics i: rate law and activation energy name date pre lab questions 1. the following rate data were collected for the reaction at 100°c 2 no, f2 2 nof no] (m) 0.0480 0.0120 0.0480 [f,] (m) 0.0317 0.0317 0.127 rate (m s) 0.00190 0.000469 0.00757 2 3 determine the reaction order for no and f2 a. b. Experiment #8 activation energy in chemical kinetics department of chemistry and chemical technology chemistry of solutions, 202 nyb 05, section: 04, winter semester 2020 farbod niazi 1934663 partner: arshia tehrani mehr 1934719 dr. rodney s.t. squire performed on may 11, 2020 submitted on may 18, 2020. View lab chemistry ii lab 9.docx from chmy 202 nyb 05 at dawson college. activation energy in chemical kinetics imane karkachi, 1832114 tristan carrière 202 nyb 05, sect. 00013, roger. Activation energy in chemical kinematics adrian pequegnat 1. record concentrations of ki, na2s2o3, (nh4)2s2o8, and (nh4)2so4. 2. follow the procedure for run 9 of the experiment "the rate law in chemical kinetics." 3. prepare flask 1 with 30.0 ml ki and 15.0 ml na2s2o3. flask 2 contains 10.0 ml (nh4)2s2o8, 20.0 ml (nh4)2so4, and 2 3 drops of.

Chemistry 30 Chemical Kinetics Activation Energy View lab chemistry ii lab 9.docx from chmy 202 nyb 05 at dawson college. activation energy in chemical kinetics imane karkachi, 1832114 tristan carrière 202 nyb 05, sect. 00013, roger. Activation energy in chemical kinematics adrian pequegnat 1. record concentrations of ki, na2s2o3, (nh4)2s2o8, and (nh4)2so4. 2. follow the procedure for run 9 of the experiment "the rate law in chemical kinetics." 3. prepare flask 1 with 30.0 ml ki and 15.0 ml na2s2o3. flask 2 contains 10.0 ml (nh4)2s2o8, 20.0 ml (nh4)2so4, and 2 3 drops of.
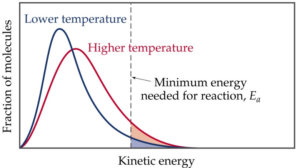
Activation Energy In Chemical Kinetics W3schools

Comments are closed.