Algernon Gets Notice Of Allowance For Patent To Treat Chronic Kidney Disease In Japan

Algernon Gets Notice Of Allowance For Patent To Treat Chronic Kidney Vancouver, british columbia, may 31, 2023 (globe newswire) algernon pharmaceuticals inc. (cse: agn) (frankfurt: agw0) (otcqb: agnpf) (the “company” or “algernon”), a clinical stage pharmaceutical development company, is pleased to announce that it has received a notice of allowance from the japanese patent office (jpo) for patent application 2021 522114 entitled “compositions and. The company was recently issued patents for repirinast in chronic kidney disease (ckd) from japan and has also filed corresponding patent applications in canada, europe and the united states. repirinast is the company’s lead candidate for the treatment of ckd based on data showing it reduced fibrosis by 51% with statistical significance and.
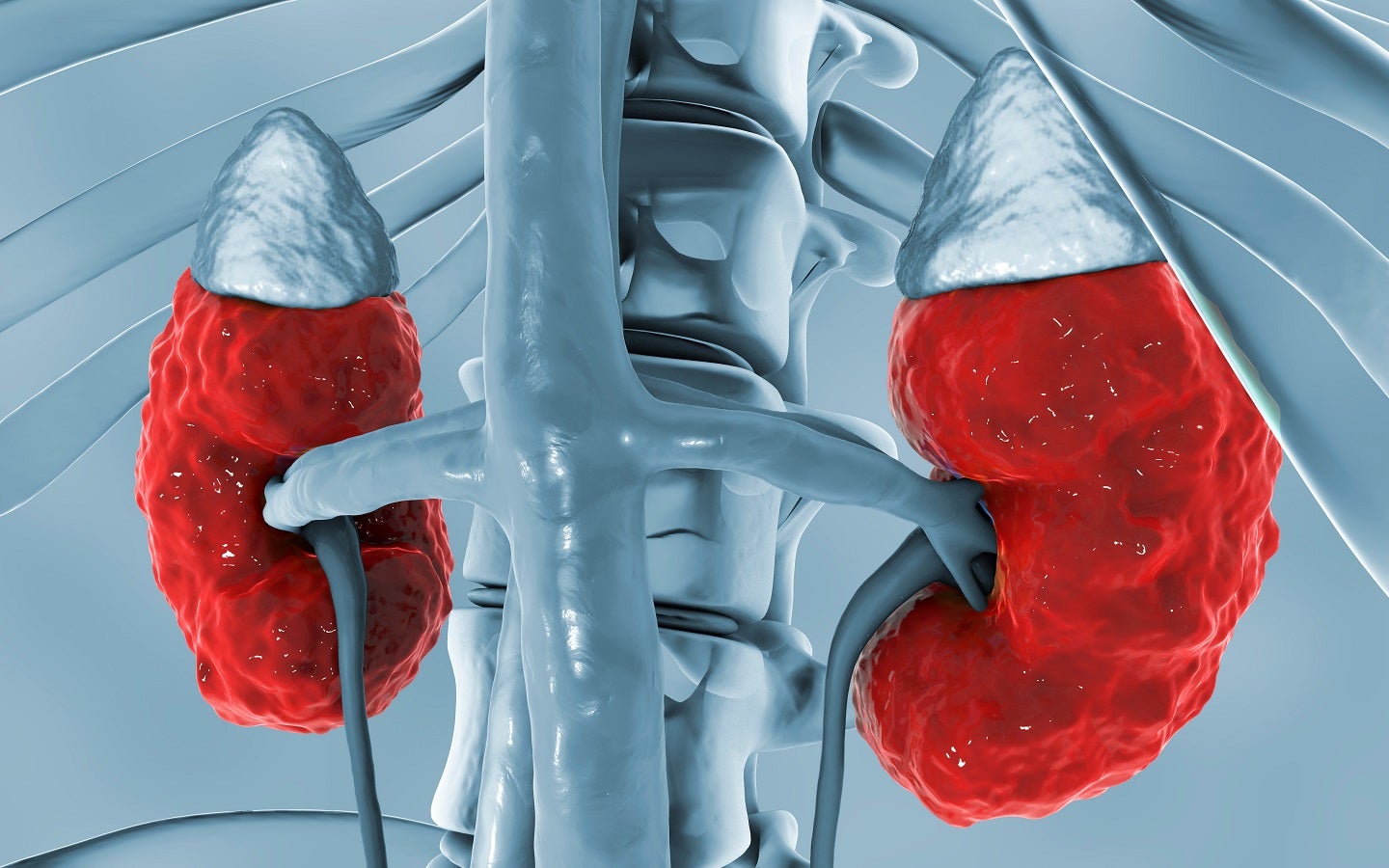
Algernon Announces Acceptance Of Repirinast Patent Application Vancouver, british columbia, jan. 11, 2024 (globe newswire) algernon pharmaceuticals inc. (the “company” or “algernon”) (cse: agn) (frankfurt: agw0) (otcqb: agnpf) a canadian clinical stage pharmaceutical development company is pleased to announce that it has received a notice of allowance from the japanese patent office for patent application no. 2021 547495 entitled: compositions. Vancouver, british columbia, may 31, 2023 (globe newswire) algernon pharmaceuticals inc. (cse: agn) (frankfurt: agw0) (otcqb: agnpf) (the “company” or “algernon”), a clinical stage. Algernon pharmaceuticals inc. is a clinical stage drug development company. the company is focused on developing repurposed therapeutic drugs in the areas of non alcoholic steatohepatitis (nash), a type of liver disease, chronic kidney disease (ckd), inflammatory bowel disease (ibd), idiopathic pulmonary fibrosis (ipf) and chronic cough as well as advancing a stroke program using n, n. Algernon pharmaceuticals has active research programs for ipf with chronic cough, and chronic kidney disease, and is the parent company of a newly created private subsidiary called algernon.

Algernon Pharmaceuticals Secures Patent For Promising Chronic Kidney Algernon pharmaceuticals inc. is a clinical stage drug development company. the company is focused on developing repurposed therapeutic drugs in the areas of non alcoholic steatohepatitis (nash), a type of liver disease, chronic kidney disease (ckd), inflammatory bowel disease (ibd), idiopathic pulmonary fibrosis (ipf) and chronic cough as well as advancing a stroke program using n, n. Algernon pharmaceuticals has active research programs for ipf with chronic cough, and chronic kidney disease, and is the parent company of a newly created private subsidiary called algernon. Vancouver, british columbia, april 17, 2023 (globe newswire) algernon pharmaceuticals inc. (the “company” or “algernon”) (cse: agn) (frankfurt: agw0) (otcqb: agnpf), a clinical stage pharmaceutical development company, is pleased to announce that it has received a notice of allowance from the united states patent and trademark office (uspto) for patent application 17 258,402. Algernon pharmaceuticals ceo christopher moreau joined steve darling from proactive to share news the company has been granted a patent in japan for its work around the compositions and methods for treating chronic kidney disease with drug np 251 or repirinast.

Algernon Pharmaceuticals Announces Notice Of Allowance For Method Of Vancouver, british columbia, april 17, 2023 (globe newswire) algernon pharmaceuticals inc. (the “company” or “algernon”) (cse: agn) (frankfurt: agw0) (otcqb: agnpf), a clinical stage pharmaceutical development company, is pleased to announce that it has received a notice of allowance from the united states patent and trademark office (uspto) for patent application 17 258,402. Algernon pharmaceuticals ceo christopher moreau joined steve darling from proactive to share news the company has been granted a patent in japan for its work around the compositions and methods for treating chronic kidney disease with drug np 251 or repirinast.

Comments are closed.