Arrhenius Equation Activation Energy And Rate Constant K Explained
Arrhenius Equation Activation Energy And Rate Constant K Explained Calculate the activation energy if the pre exponential factor is 15 m 1 s 1, rate constant is 12m 1 s 1 and it is at 22k; find the new temperature if the rate constant at that temperature is 15m 1 s 1 while at temperature 389k the rate constant is 7m 1 s 1, the activation energy is 600kj mol. Figure 6.2.3.3.1: lowering the activation energy of a reaction by a catalyst. this graph compares potential energy diagrams for a single step reaction in the presence and absence of a catalyst. the only effect of the catalyst is to lower the activation energy of the reaction. the catalyst does not affect the energy of the reactants or products.

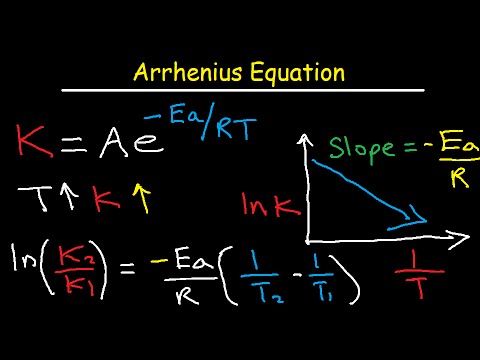
Collision Theory Arrhenius Equation Activation Energy Chemical The gas constant, r. this is a constant which comes from an equation, pv=nrt, which relates the pressure, volume and temperature of a particular number of moles of gas. it turns up in all sorts of unlikely places! activation energy, ea. this is the minimum energy needed for the reaction to occur. This chemistry video tutorial focuses on the arrhenius equation and how to derive it's many different forms within the subject of chemical kinetics. activat. The activation energy of a chemical reaction is 100 kj mol and it’s a factor is 10 m 1 s 1. find the rate constant of this equation at a temperature of 300 k. given, e a = 100 kj.mol 1 = 100000 j.mol 1. a = 10 m 1 s 1, ln(a) = 2.3 (approx.) t = 300 k. the value of the rate constant can be obtained from the logarithmic form of the arrhenius. Both the arrhenius activation energy and the rate constant k are experimentally determined, and represent macroscopic reaction specific parameters that are not simply related to threshold energies and the success of individual collisions at the molecular level. consider a particular collision (an elementary reaction) between molecules a and b.

Rate Constants And The Arrhenius Equation The activation energy of a chemical reaction is 100 kj mol and it’s a factor is 10 m 1 s 1. find the rate constant of this equation at a temperature of 300 k. given, e a = 100 kj.mol 1 = 100000 j.mol 1. a = 10 m 1 s 1, ln(a) = 2.3 (approx.) t = 300 k. the value of the rate constant can be obtained from the logarithmic form of the arrhenius. Both the arrhenius activation energy and the rate constant k are experimentally determined, and represent macroscopic reaction specific parameters that are not simply related to threshold energies and the success of individual collisions at the molecular level. consider a particular collision (an elementary reaction) between molecules a and b. Now that we have obtained the activation energy and pre exponential factor from the arrhenius plot, we can solve for the rate constant at any temperature using the arrhenius equation. the arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1 t. In 1889, a swedish scientist named svante arrhenius proposed an equation that relates these concepts with the rate constant: where k represents the rate constant, ea is the activation energy, r is the gas constant , and t is the temperature expressed in kelvin. a is known as the frequency factor, having units of l mol −1 s −1, and takes.

Comments are closed.