Atomic Number Isotopes And Isobars Definition Examples And Faqs Of

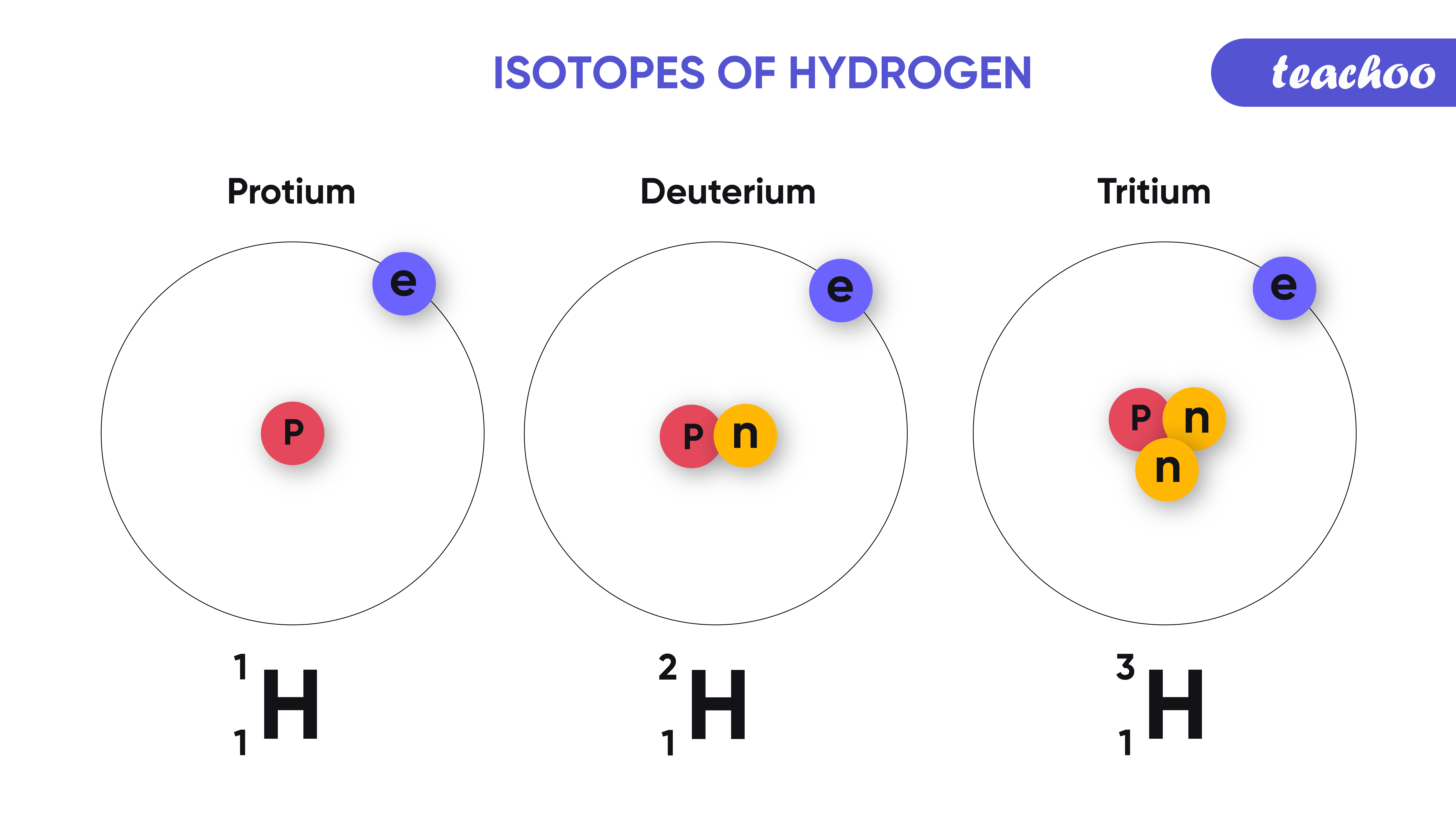
Atomic Number Isotopes And Isobars Definition Examples And Faqs Of Atomic number, isotopes and isobars atomic number of an element is the total number of protons present in that element, mass number is the sum of the number of protons and neutrons of that element. the atoms having the same atomic number but different mass number are called isotopes. to learn more about the atomic number, isotopes and isobars, its definition, examples and faqs, visit byju. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. an example of two isotopes and isobars is nickel and iron. these both have the same mass number, which is 58, whereas the atomic number of nickel is 28, and the atomic number of iron is 26.

Atomic Number Isotopes And Isobars Definition Examples And Faqs Of The word “isobar” has been derived from greek word whose meaning is “ equally heavy ” (isos = equal and barys = heavy). for example, carbon 14 (146 c) and nitrogen 14 (147 n) are isobars in chemistry. as you can observe in this example, these atoms have the same mass number 14, but different atomic numbers, which are 6 and 7. Chemistry. isotopes are elements with the same atomic number but distinct mass numbers. isobars are elements with various atomic numbers but the same mass number. an example of two isotopes and isobars is nickel and iron. Isotopes and isobars. isobar are elements that differ in chemical properties but have the same physical property. so, we can say that isobars are those elements that have a different atomic number but the same mass number. in contrast, isotopes are those elements having the same atomic number and different mass numbers. The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements the concept of the nucleus was discovered by rutheford in his atomic model popularly known as rutherford’s atomic model, which states that ‘protons and neutrons, which constitute almost all of the mass of the nuclear atom, are found in the nucleus and lies in the.

Isotopes And Isobars Definition Uses And Difference Teachoo Isotopes and isobars. isobar are elements that differ in chemical properties but have the same physical property. so, we can say that isobars are those elements that have a different atomic number but the same mass number. in contrast, isotopes are those elements having the same atomic number and different mass numbers. The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements the concept of the nucleus was discovered by rutheford in his atomic model popularly known as rutherford’s atomic model, which states that ‘protons and neutrons, which constitute almost all of the mass of the nuclear atom, are found in the nucleus and lies in the. Sometimes, the atomic masses of different elements can be the same although their atomic numbers are different from each other. these different forms are called isobars. the main difference between isotopes and isobars is that the atomic masses of isotopes are different from each other whereas the atomic masses of isobars are similar to each other. Isobars are atoms of different objects that have the same number of numbers but different atomic numbers. for example, two elements of calcium and argon. the number of electrons in these atoms varies, but the maximum number of the two is 40.17. isobars are types of atoms that have the same number of masses (a), but a different number of atoms (z).

Atomic Number Isotopes And Isobars Definition Examples And Faqs Of Sometimes, the atomic masses of different elements can be the same although their atomic numbers are different from each other. these different forms are called isobars. the main difference between isotopes and isobars is that the atomic masses of isotopes are different from each other whereas the atomic masses of isobars are similar to each other. Isobars are atoms of different objects that have the same number of numbers but different atomic numbers. for example, two elements of calcium and argon. the number of electrons in these atoms varies, but the maximum number of the two is 40.17. isobars are types of atoms that have the same number of masses (a), but a different number of atoms (z).
Isotopes And Isobars Definition Uses And Difference Teachoo

Comments are closed.