Binodal Curve Tcb Binodal Curve C Critical Point Tb Tie Line

Binodal Curve Tcb Binodal Curve C Critical Point Tb Tie Line Note that the relationship among the concentrations of the components is more complex than that of binary systems. figure 5.4.1 5.4. 1: three component triangular representation (i.e. 'blank graph paper' for a ternary diagram phase diagram), with axis labels perpendicular to each plotting axis to facilitate plotting and comprehension. The line (tb) in the diagram (see fig. 1) is a tie line; it connects two nodes, which lie on the binodal curve. all the potential systems (e.g., s1, s2, s3) have same top phase and bottom phase equilibrium composition be cause of being on the same tie line. point c on binodal is called as a critical point, just above this point the vol.

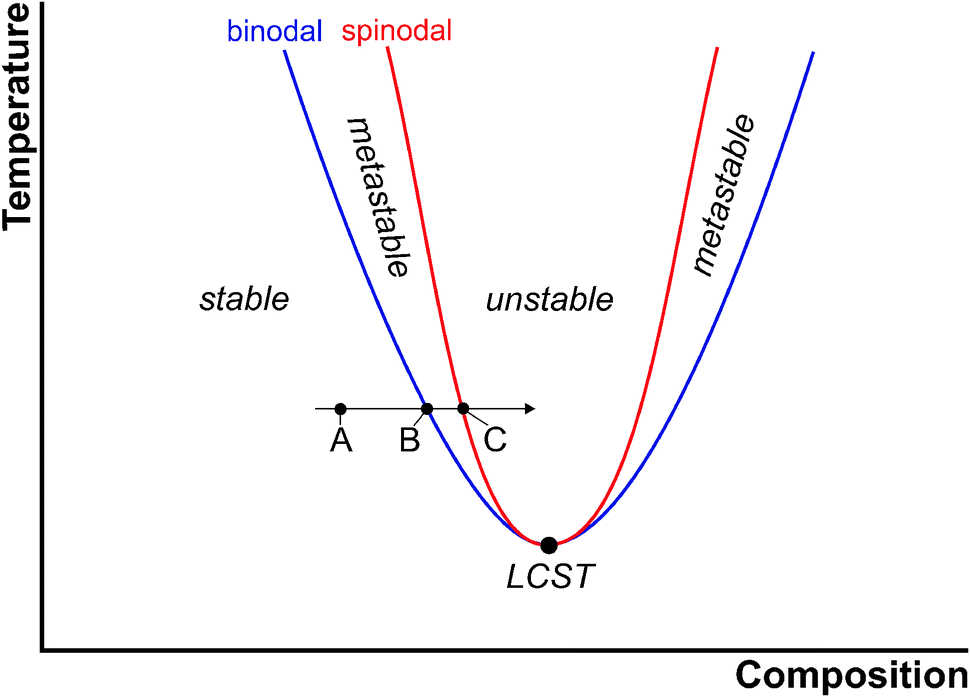
Binodal Curve In The Figure Tcb Binodal Curve C Critical Point Binodal curve, tcb = binodal curve, c = critical point, tb = tie line, t = composition of the top phase, b = composition of the bottom phase, and x, y and z = total composition of atps. Binodal curve. in the figure, tcb = binodal curve, c = critical point, tb = tie line, t = composition of the top phase, b = composition of the bottom phase, and x, y and z = total composition of atps. There is no solution for c for χ <χ < 2 2 so there is no phase separation. also, for the symmetrical case, the common tangent construction reduces to the condition df dc = 0 (i.e. horizontal tangent) this equation defines the binodal or coexistence curve. χbinodal = 1 2c−1 ln(c 1−c) for the regular solution case, with χ∝ 1 Τ this allows. Referred to as the binodal or coexistence curve, is found by performing a common tangent construction of the free energy diagram. inside the binodal is a region called the spinodal, which is found by determining where the curvature of the free energy curve is negative. the binodal and spinodal meet at the critical point.

General Two Phase System Binodal With Critical Point C Tie Lines There is no solution for c for χ <χ < 2 2 so there is no phase separation. also, for the symmetrical case, the common tangent construction reduces to the condition df dc = 0 (i.e. horizontal tangent) this equation defines the binodal or coexistence curve. χbinodal = 1 2c−1 ln(c 1−c) for the regular solution case, with χ∝ 1 Τ this allows. Referred to as the binodal or coexistence curve, is found by performing a common tangent construction of the free energy diagram. inside the binodal is a region called the spinodal, which is found by determining where the curvature of the free energy curve is negative. the binodal and spinodal meet at the critical point. In thermodynamics, the binodal, also known as the coexistence curve or binodal curve, denotes the condition at which two distinct phases may coexist. equivalently, it is the boundary between the set of conditions in which it is thermodynamically favorable for the system to be fully mixed and the set of conditions in which it is. Figure 5.7: ternary phase diagram for a ternary system. the binodal curve is formed of the bubble point curve and the dew point curve, both of which meet at the plait point. this is the point at which the liquid and vapor composition are identical (resembles the critical point that we studied before). within the two phase region, the tie lines.

General Two Phase System Binodal With Critical Point C Tie Lines In thermodynamics, the binodal, also known as the coexistence curve or binodal curve, denotes the condition at which two distinct phases may coexist. equivalently, it is the boundary between the set of conditions in which it is thermodynamically favorable for the system to be fully mixed and the set of conditions in which it is. Figure 5.7: ternary phase diagram for a ternary system. the binodal curve is formed of the bubble point curve and the dew point curve, both of which meet at the plait point. this is the point at which the liquid and vapor composition are identical (resembles the critical point that we studied before). within the two phase region, the tie lines.
Binodal

Comments are closed.