Biosimilar And Interchangeable Biologics Rxtoolkit

Biosimilar And Interchangeable Biologics вђ Rxtoolkit The Federal Trade Commission submitted a comment supporting the Food and Drug Administration’s draft guidance regarding interchangeable biosimilar the goals of the Biologics Price Over the next 10– 15 years, the biosimilars industry is expected to explode as the patents on branded biologics begin to expire Analysts agree that biosimilar market size will be fairly
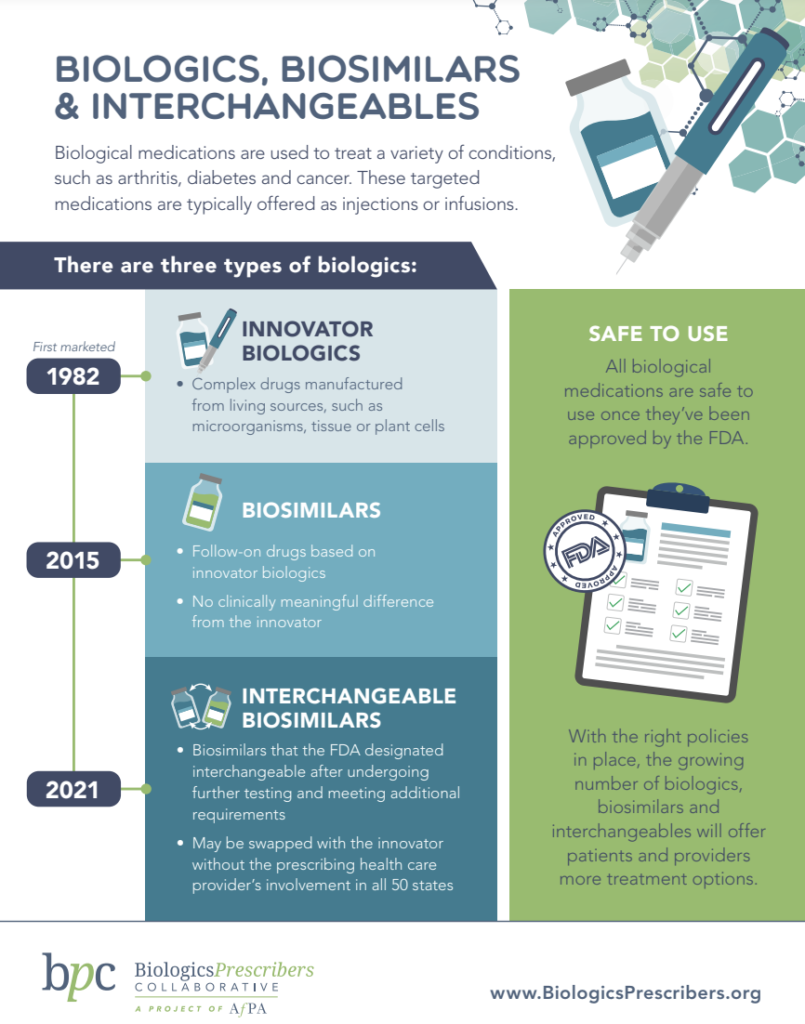
Biologics Biosimilars Interchangeables Biologics Prescribers We recently posted on a new FDA draft guidance entitled “Postapproval Manufacturing Changes to Biosimilar and Interchangeable and innovation among biologics, [and] could lead to lower President Joe Biden’s budget proposal for fiscal 2025 includes a proposal that would allow pharmacy substitution of biosimilar authority to designate biologics as interchangeable was created Biologics are granted 12 years of exclusivity -- not including additional patent protection -- shielding them from potential competition For traditional small molecule drugs, it is only five The Biosimilars Council recently commented on the FDA’s June 2024 Draft Guidance Docket No FDA–2017-D-0154, which is focused on considerations regarding a switching study or studies intended

Comments are closed.