Bond Formation In Oxygen Molecule Photograph By Animate4 Science
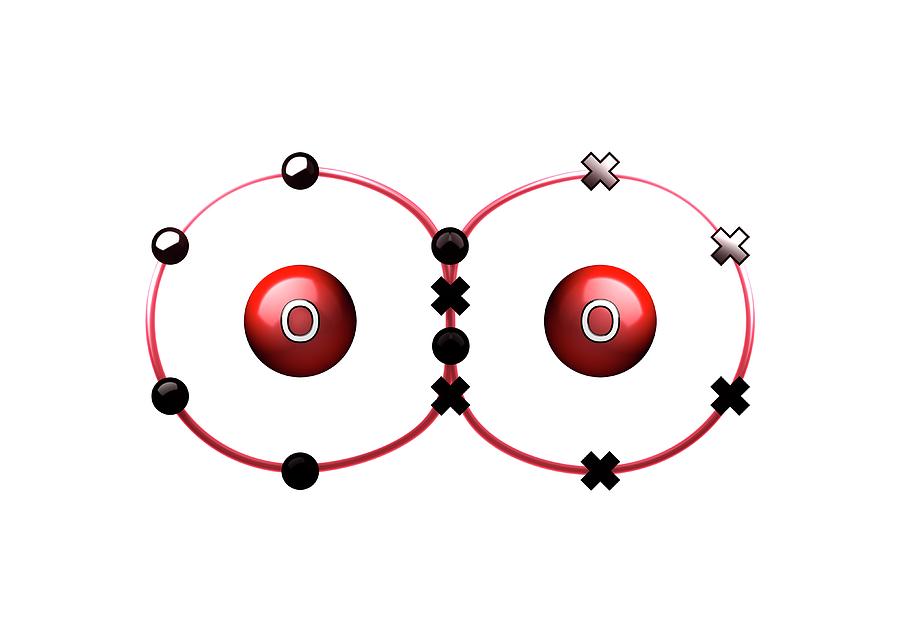
Bond Formation In Oxygen Molecule Photograph By Animate4 Science Caption. bond formation in oxygen molecule. animation of the sharing of electrons (dots and crosses) between two oxygen atoms (o) to form a molecule of oxygen (o2). this is an example of covalent bonding, with the double bond shown here formed by two shared electron pairs, each pair consisting of an electron from each atom. Purchase an art print of the photograph "bond formation in oxygen molecule" by science photo library. choose from multiple sizes and hundreds of frame and mat options. all prints are professionally printed, packaged, and shipped within 3 4 business days.

Bond Formation Photograph By Animate4 Science Photo Libary Pixels In the final steps before o=o bond formation, a new oxygen, o6, binds to the vacant site at mn1. after a final photo oxidation event, o5 and o6 appear poised to form an o=o bond, releasing molecular oxygen, reducing the cluster, and beginning the catalytic cycle anew. glutamatic acid at position 189 is noted as e189. An oxygen atom has 6 electrons in its outer shell. oxygen is in group 6 of the periodic table. two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule (o 2). this is a picture of an oxygen molecule. by sharing the four electrons where the shells touch each oxygen atom can count 8 electrons in its. (2) if the p y orbital of one 'o' atom overlaps the py orbital of other 'o' atom along internuclear axis, a σ p y – p y bond is formed. (3) p z orbital of oxygen atom overlap laterally, perpendicular to inter nuclear axis giving a π p z – p z bond. (4) so o 2 molecule has a double bond between the two oxygen atoms. Upon the last oxidation of y z, (forming the s 3 y z • state), the system rapidly decays to the s 0 state, with the concomitant release of molecular triplet oxygen and the rebinding of one substrate water molecule (4, 5). the chemical steps involved in o o bond formation in the transition state (s 4) remain uncertain.

Bond Formation In Oxygen Molecule Stock Video Clip K004 5697 (2) if the p y orbital of one 'o' atom overlaps the py orbital of other 'o' atom along internuclear axis, a σ p y – p y bond is formed. (3) p z orbital of oxygen atom overlap laterally, perpendicular to inter nuclear axis giving a π p z – p z bond. (4) so o 2 molecule has a double bond between the two oxygen atoms. Upon the last oxidation of y z, (forming the s 3 y z • state), the system rapidly decays to the s 0 state, with the concomitant release of molecular triplet oxygen and the rebinding of one substrate water molecule (4, 5). the chemical steps involved in o o bond formation in the transition state (s 4) remain uncertain. Hence, the bond order of oxygen molecule is 2. oxygen molecule has two bonds. i.e., one is σ bond and one π π π bond. the last 2 electrons in p orbital acts as lone pair of electrons. the presence of unpaired electron in oxygen atom shows its paramagnetic nature. suggest corrections. Bond formation in oxygen molecule is a photograph by animate4 science photo libary which was uploaded on august 4th, 2016. the photograph may be purchased as wall.

Vector Diagram Formation Oxygen Molecule Stock Vector Royalty Free Hence, the bond order of oxygen molecule is 2. oxygen molecule has two bonds. i.e., one is σ bond and one π π π bond. the last 2 electrons in p orbital acts as lone pair of electrons. the presence of unpaired electron in oxygen atom shows its paramagnetic nature. suggest corrections. Bond formation in oxygen molecule is a photograph by animate4 science photo libary which was uploaded on august 4th, 2016. the photograph may be purchased as wall.

Comments are closed.