Cl2 Lewis Structure Molecular Shape Polar Or Non Polar Dot Diagram

Cl2 Lewis Structure Molecular Shape Polar Or Non Polar Dot Diagram The cl cl bond is non polar since the electronegativity difference is zero. the dipole moment of the bond comes out to be zero. in a diatomic molecule, a non polar bond implies a non polar molecule. must read: is cl2 polar or nonpolar. is n2 polar or nonpolar . preparation of chlorine gas . heating concentrated hcl with metal oxide. Cl2 is the diatomic form of chlorine gas, a strong oxidising agent and a greenish yellow gas. the lewis dot structure for cl2 demonstrates how a single covalent bond between the chlorine atoms creates a stable octet of electrons around each chlorine atom.

Cl2 Lewis Structure Molecular Shape Polar Or Non Polar Dot Diagram The total valence electron available for drawing the cl2 lewis structure is 14. the hybridization of each chlorine atom in cl2 is sp³. the formal charge of chlorine in the cl2 lewis dot structure is zero. the molecular shape of cl2 is linear. cl2 is non polar in nature because of no dipole moment present in it. Bf 3 is a trigonal planar molecule and all three peripheral atoms are the same. figure 4.12.1 4.12. 1 some examples of nonpolar molecules based on molecular geometry (bf 3 and ccl 4). polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. Tetrahedral, non polar b. ncl 3 trigonal pyramidal, polar c. ccl 2 f 2 tetrahedral, polar d. cf 2 h 2 tetrahedral, polar e. ch 2 o trigonal planar, polar f. chn linear, polar g. pi 3 trigonal pyramidal, polar h. n 2 o linear, polar i. so 2 bent, polar j. cs 2 linear, non polar k. co linear, polar l. h 2 o bent, polar m. cof 2 trigonal planar. A step by step explanation of how to draw the cl2 lewis dot structure (chlorine gas).for the cl2 structure use the periodic table to find the total number of.

Cl2 Lewis Structure Molecular Shape Polar Or Non Polar Dot Diagram Tetrahedral, non polar b. ncl 3 trigonal pyramidal, polar c. ccl 2 f 2 tetrahedral, polar d. cf 2 h 2 tetrahedral, polar e. ch 2 o trigonal planar, polar f. chn linear, polar g. pi 3 trigonal pyramidal, polar h. n 2 o linear, polar i. so 2 bent, polar j. cs 2 linear, non polar k. co linear, polar l. h 2 o bent, polar m. cof 2 trigonal planar. A step by step explanation of how to draw the cl2 lewis dot structure (chlorine gas).for the cl2 structure use the periodic table to find the total number of. In the above structure, you can see that the central atom (right chlorine) forms an octet. and the outside atom (left chlorine) also forms an octet. hence, the octet rule is satisfied. therefore, this structure is the stable lewis structure of cl 2. next: hf lewis structure your feedback matters. visit our contact page. Fluorine (f 2) and bromine (br 2) are similar lewis structures to chlorine molecule considering the structure of bonds between atoms and number of lone pairs around atoms though, chlorine molecule is a non polar molecule, chlorine gives an acidic solution when chlorine gas is dissolved in water. please explain the reason.

Cl2 Lewis Dot Structure In the above structure, you can see that the central atom (right chlorine) forms an octet. and the outside atom (left chlorine) also forms an octet. hence, the octet rule is satisfied. therefore, this structure is the stable lewis structure of cl 2. next: hf lewis structure your feedback matters. visit our contact page. Fluorine (f 2) and bromine (br 2) are similar lewis structures to chlorine molecule considering the structure of bonds between atoms and number of lone pairs around atoms though, chlorine molecule is a non polar molecule, chlorine gives an acidic solution when chlorine gas is dissolved in water. please explain the reason.
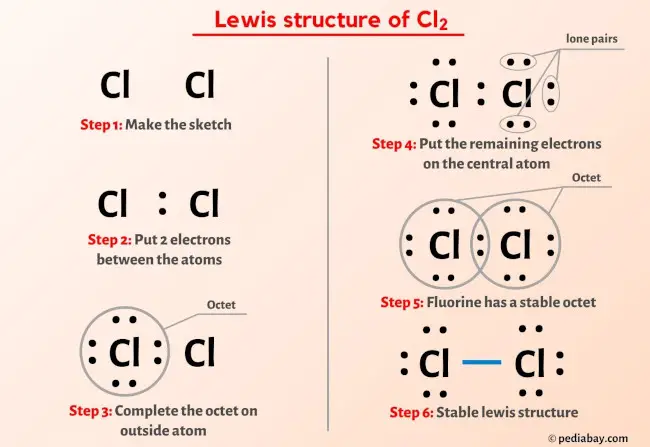
Lewis Structure Of Cl2

Comments are closed.