Cl2 Lewis Structure Valence Electrons Formal Charge Polar Or Nonpolar

Cl2 Lewis Structure Valence Electrons Formal Charge Polar Or Nonpolar Formal charge= (valence electrons in isolate state of a neutral atom) – (non bonding valence electrons on atom) – (number of bonds formed by atom) steps to draw the lewis structure of cl2. 1. count the total number of valence shell electrons on the molecule . this is done by adding the valence shell electrons of all the constituent atoms. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best lewis structure. we already have the best lewis structure for cl 2. 7. check the stability of the structure. it can be checked by using the formula formal charge = valence electrons – unbonded electrons – ½ bonded electrons.

Cl2 Molecular Geometry Valence electrons of two chlorine atoms = 7 × 2 = 14. so the total valence electrons = 14. learn how to find: chlorine valence electrons. second, find the total electron pairs; we have a total of 14 valence electrons. and when we divide this value by two, we get the value of total electron pairs. total electron pairs = total valence electrons ÷ 2. Here is the formula for determining the formal charge of each atom in cl2 lewis structure. •formal charge =valence electrons unshared electrons 1 2 (shared electrons) •valence electrons of cl =7. •shared electrons of cl= 2. •unshared electrons of cl = 6. f.c. of cl =7 6 1 2 (2) =7 6 1. Here are the steps to draw the correct and stable lewis structure: 1. determine the total number of valence electrons in the chlorine molecule. there are two chlorine atoms in the chlorine molecule. each chlorine atom, as a group viia element in the periodic table, has seven electrons in its outer shell. therefore, the total number of valence. Torrey glenn, city college of san francisco. 9.3: drawing lewis structures is shared under a license and was authored, remixed, and or curated by libretexts. a lewis structure is a way to show how atoms share electrons when they form a molecule. lewis structures show all of the valence electrons in an atom or molecule.
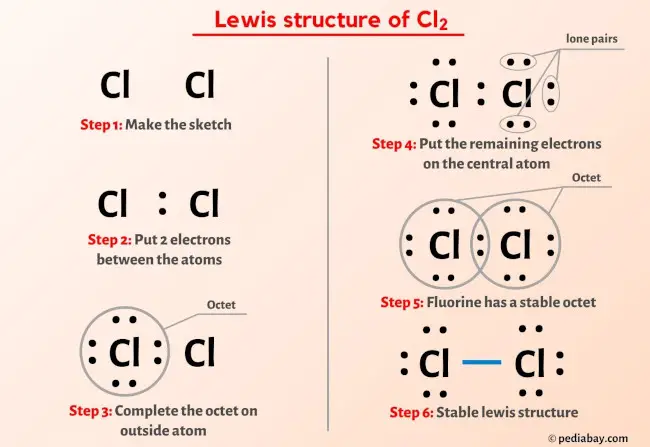
Cl2 Lewis Diagram Here are the steps to draw the correct and stable lewis structure: 1. determine the total number of valence electrons in the chlorine molecule. there are two chlorine atoms in the chlorine molecule. each chlorine atom, as a group viia element in the periodic table, has seven electrons in its outer shell. therefore, the total number of valence. Torrey glenn, city college of san francisco. 9.3: drawing lewis structures is shared under a license and was authored, remixed, and or curated by libretexts. a lewis structure is a way to show how atoms share electrons when they form a molecule. lewis structures show all of the valence electrons in an atom or molecule. 2. each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 3. placing one bonding pair of electrons between the o atom and each h atom gives h:o:h, with 4 electrons left over. 4. each h atom has a full valence shell of 2 electrons. Figure \(\pageindex{8}\): some examples of polar and nonpolar molecules based on molecular geometry. to summarize, to be polar, a molecule must: contain at least one polar covalent bond. have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel. steps to identify polar molecules. draw the lewis structure.

Comments are closed.