Consumer Product Labeling Requirements Usa

Understanding Fda Labeling Requirements For Food Products Summary of most common u.s. cpsc labeling requirements: tracking labels. all children’s products must be labeled with tracking information on the product and its packaging, if practicable to do so. small parts. the specific small parts labeling requirements for products containing small parts and intended for children between the ages of 3. The fair packaging and labeling act (fpla or act), enacted in 1967, directs the federal trade commission and the food and drug administration to issue regulations requiring that all "consumer commodities" be labeled to disclose net contents, identity of commodity, and name and place of business of the product's manufacturer, packer, or distributor.

United States Product Labeling Requirements A Complete Guide Products sold in the united states must generally be labeled or marked according to the requirements in the applicable regulations. some labeling requirements apply to all products, while others only cover certain types of products, materials, or even age groups. commonly, a single product is subject to several different product labeling and. Section 2 (q) (1) (b) of the fhsa, 15 u.s.c. § 1261 (q) (1) (b), allows the commission to ban certain products that are so dangerous, or the nature of the hazard is such that precautionary labeling is not adequate to protect consumers. such products are listed at 16 c.f.r. § 1500.17. petitions for exemptions from full labeling requirements or. This section describes the general labeling requirements for all packaged goods sold by weight, volume, count or measure (e.g., length, width, and thickness). additional guidance on other requirements, conversion factors, and information is presented in later sections. specific requirements for quantity declarations are found in part iii. The following exemptions are granted from label statements required by this part: (a) foods. (1) while held for sale, a food shall be exempt from the required declaration of net quantity of contents specified in this part if said food is received in bulk containers at a retail establishment and is accurately weighed, measured, or counted either.

Usa Food Labeling Regulations Fdabasics This section describes the general labeling requirements for all packaged goods sold by weight, volume, count or measure (e.g., length, width, and thickness). additional guidance on other requirements, conversion factors, and information is presented in later sections. specific requirements for quantity declarations are found in part iii. The following exemptions are granted from label statements required by this part: (a) foods. (1) while held for sale, a food shall be exempt from the required declaration of net quantity of contents specified in this part if said food is received in bulk containers at a retail establishment and is accurately weighed, measured, or counted either. As used in this part, unless the context otherwise specifically requires: (a) the term act means the “fair packaging and labeling act” (pub. l. 89 755, approved nov. 3, 1966; 80 stat. 1296 et seq.; 15 u.s.c. 1451 et seq., as amended by public law 102 329, august 3, 1992). (b) the term regulation or regulations means regulations promulgated. The fair packaging and labeling act (fpla) and other federal laws and regulations govern the labeling requirements for most consumer products; however, many products fall only under state laws (nist handbook 130 current edition). over the years, packaging and labeling laws and regulations have been introduced and modified to meet marketplace needs.
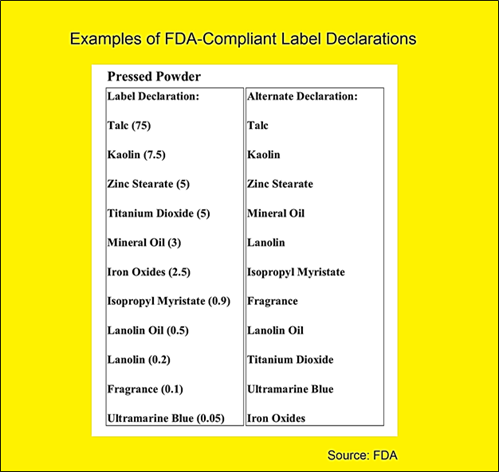
Fda Cosmetic Labeling Requirements And Label Printing Guide As used in this part, unless the context otherwise specifically requires: (a) the term act means the “fair packaging and labeling act” (pub. l. 89 755, approved nov. 3, 1966; 80 stat. 1296 et seq.; 15 u.s.c. 1451 et seq., as amended by public law 102 329, august 3, 1992). (b) the term regulation or regulations means regulations promulgated. The fair packaging and labeling act (fpla) and other federal laws and regulations govern the labeling requirements for most consumer products; however, many products fall only under state laws (nist handbook 130 current edition). over the years, packaging and labeling laws and regulations have been introduced and modified to meet marketplace needs.

Comments are closed.