Cooling Curve Of Pure Iron Diagram
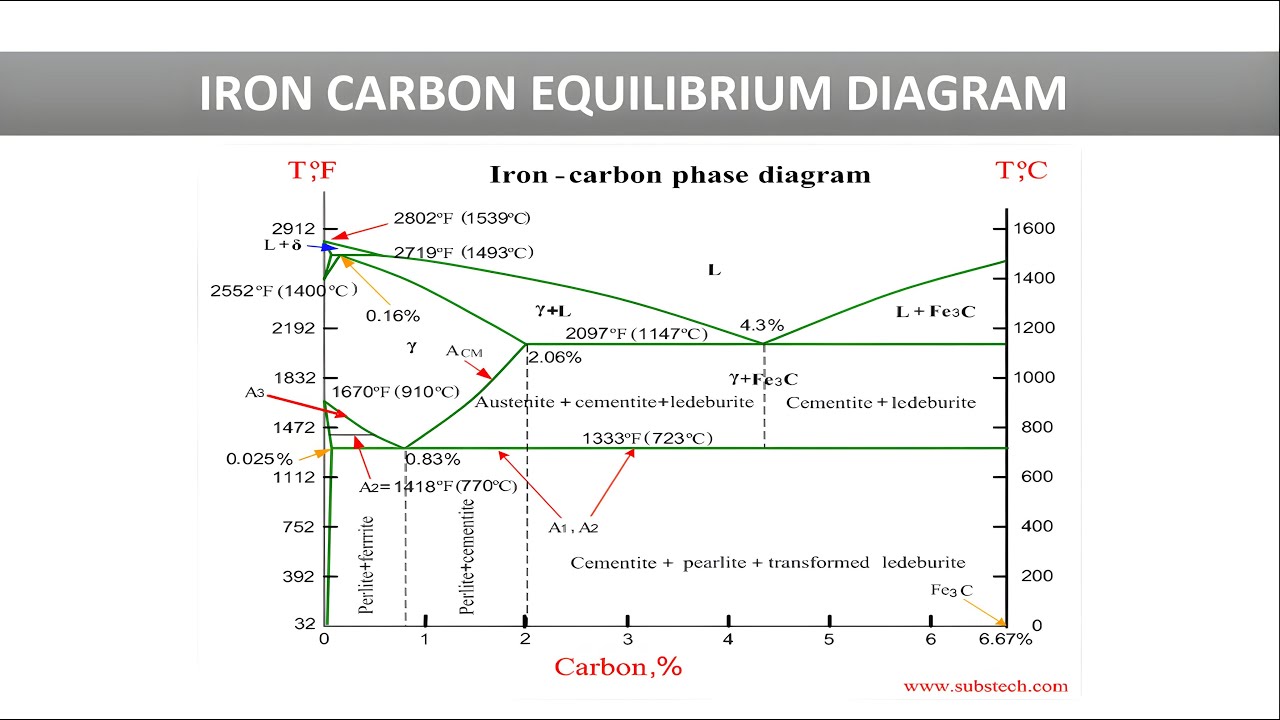
Iron Carbon Equilibrium Diagram Cooling Curve Of Pure Iron Iron By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. Therefore, the phase diagram of the iron carbon alloy system is somewhat more complex. in order to understand the microstructural processes inside a steel, it makes sense to first take a closer look at the microstructure formation of pure iron. for this reason, the cooling curve of iron is discussed in more detail in the following section.
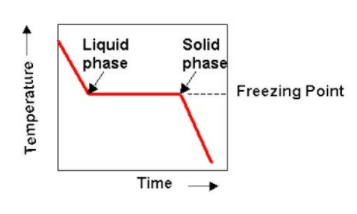
Cooling Curve For Pure Iron Marinerspoint Pro Fig 1 shows the cooling curve for pure iron. pure iron exist in the following allotropic and phases forms: α fe( α ferrite) is bcc solid solution, max solubility of c is 0.022 wt %,rt solubility of c is 0.008 wt % γ fe (γ austenite) is fcc solid solution,max solubility of c is 2.14 wt%. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. The iron–iron carbide (fe–fe3c) phase diagram the sketch in slide 1 is a typical cooling curve of pure iron. solidification begins with nucleation and growth of crystals of iron at 1539°c. it is bcc (body centered cubic). at 1394°c it transforms into fcc (face centered cubic) structure. this is stable till 910°c. Fig. 8.2. cooling curve of pure iron with steady rate of heat loos 8.2. solubility of carbon in iron the carbon atom is smaller than the iron atom (the diameters are 0·154 nm and 0'256 nm, respectively) and dissolves interstitially in all three phases. the solubility in y iron is at a maximum of 1·7 % at 1130 °c,.

Cooling Curve Of Pure Iron Allotropy Of Iron Hindi Material The iron–iron carbide (fe–fe3c) phase diagram the sketch in slide 1 is a typical cooling curve of pure iron. solidification begins with nucleation and growth of crystals of iron at 1539°c. it is bcc (body centered cubic). at 1394°c it transforms into fcc (face centered cubic) structure. this is stable till 910°c. Fig. 8.2. cooling curve of pure iron with steady rate of heat loos 8.2. solubility of carbon in iron the carbon atom is smaller than the iron atom (the diameters are 0·154 nm and 0'256 nm, respectively) and dissolves interstitially in all three phases. the solubility in y iron is at a maximum of 1·7 % at 1130 °c,. Cooling curves are shown on the temperature log time plot. at the end of the cooling curve phases are shown at room temperature. variation in hardness with distance from jominy end is also shown in the diagram. for cooling curve b, at t 1 temperature minimum t 1 timing is required to nucleate pearlite as per ttt diagram in fig. 8. but material. The sketch in slide 1 is a typical cooling curve of pure iron. solidification begins with nucleation and growth of crystals of iron at 1539°c. it is bcc (body centered cubic). at 1394°c it transforms into fcc (face centered cubic) structure.

Comments are closed.