Covalent Bonding Dot And Cross Diagrams P86

Covalent Bonding Dot And Cross Diagrams P86 Youtube About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. Dot and cross diagrams for covalent compounds. covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co 2. these small molecules are known as simple molecules. small covalent molecules can be represented by dot and cross diagrams. you need to be able to describe and draw the structures of the molecules below:.

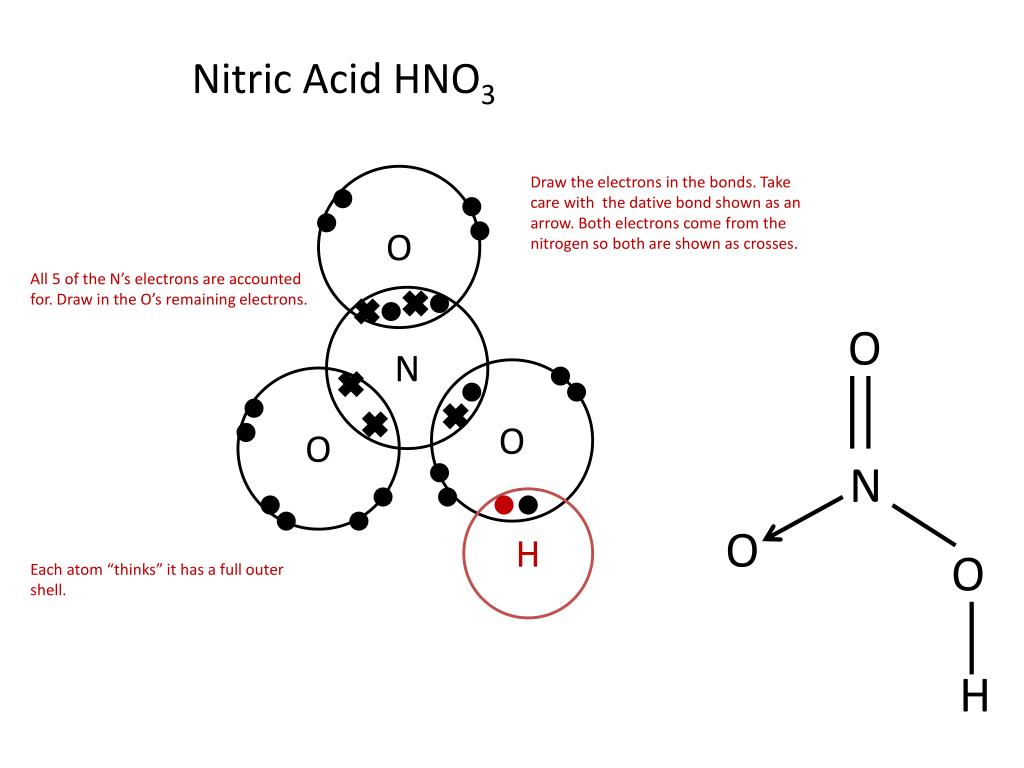
Covalent Bonding Dot Cross Diagrams Gcse Chemistry Revision Youtube An electron deficient atom is an atom that has an unfilled outer orbital. so both electrons are from the same atom. this type of bonding is called dative covalent bonding or coordinate bonding. an example with a dative bond is in an ammonium ion. the hydrogen ion, h is electron deficient and has space for two electrons in its shell. Figure 3.15.1 3.15. 1: a valid electron dot structure for sulfur. the presence of unpaired electrons within an atom is inherently destabilizing. therefore, if two atoms that contain unpaired electrons approach one another, those electrons will interact with one another to form a shared pair of electrons. the resultant covalent, or molecular. The formula is h 2 o so the dot and cross diagram includes two h atoms and one o atom. h has 1 outer electron. o has 6 outer electrons. the h circles must each overlap the o circle. nitrogen. Covalent bond the strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms. dot and cross diagram is used to show how chemical bonds are formed between atoms. the electrons from one atom are shown as dots and the electrons from the other atom are shown as crosses.
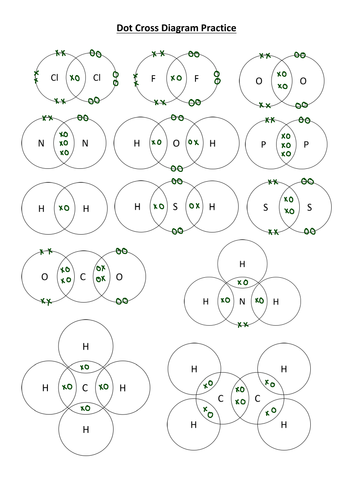
Covalent Bonding Dot Cross Diagram Worksheet With Answers Teaching The formula is h 2 o so the dot and cross diagram includes two h atoms and one o atom. h has 1 outer electron. o has 6 outer electrons. the h circles must each overlap the o circle. nitrogen. Covalent bond the strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms. dot and cross diagram is used to show how chemical bonds are formed between atoms. the electrons from one atom are shown as dots and the electrons from the other atom are shown as crosses. Draw the dot and cross diagram with the outer shells overlapping. then draw the shared electrons inside the overlapping section. nitrogen gas occurs naturally as a diatomic molecule. the bond between the two nitrogen atoms is a triple bond. again, draw the dot and cross diagram with the outer shells overlapping. A chemical bond holds the parts of a chemical structure together. when the bonding is between two non metallic atoms, a covalent bond is formed. covalently bonded species can fall into one of two categories: elements, where all the atoms are of the same non metal. compounds, where two or more different non metallic atoms are bonded together.
Ppt Showing Covalent Bonding Using Dot Cross Diagrams Powerpoint Draw the dot and cross diagram with the outer shells overlapping. then draw the shared electrons inside the overlapping section. nitrogen gas occurs naturally as a diatomic molecule. the bond between the two nitrogen atoms is a triple bond. again, draw the dot and cross diagram with the outer shells overlapping. A chemical bond holds the parts of a chemical structure together. when the bonding is between two non metallic atoms, a covalent bond is formed. covalently bonded species can fall into one of two categories: elements, where all the atoms are of the same non metal. compounds, where two or more different non metallic atoms are bonded together.

Chemical Bonds Ionic Covalent And Metallic Aqa C2 Revisechemistry Uk

Comments are closed.