Diagram Of Cooling Curve Of A Liquid Freezing Point

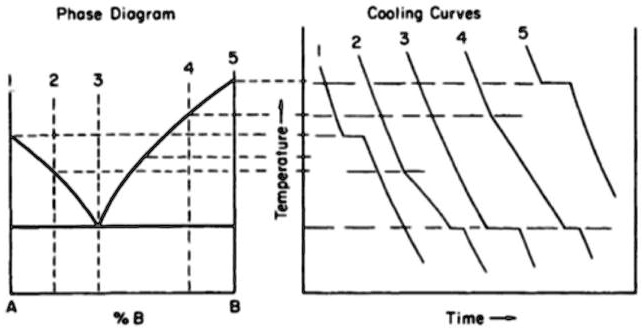
Freezing Point On Phase Diagram By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate.

Heating And Cooling Curves Overview Examples Expii The liquid must first be cooled to its freezing point (the same temperature as its melting point). a cooling curve close cooling curve a graph showing the temperature of a substance against. Figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. the change of state behavior of all substances can be represented with a heating curve of this type. The cooling curve for pure liquid tin looks like this: it's just like the pure lead cooling curve except that tin's freezing point is lower. if you add small amounts of lead to the tin, so that you have perhaps 80% tin and 20% lead, you will get a curve like this: notice the lowered freezing point of the tin. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember.

Cooling Curve Spm Chemistry The cooling curve for pure liquid tin looks like this: it's just like the pure lead cooling curve except that tin's freezing point is lower. if you add small amounts of lead to the tin, so that you have perhaps 80% tin and 20% lead, you will get a curve like this: notice the lowered freezing point of the tin. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember. An experimental cooling curve for a pure liquid is shown in figure 15 1 and for a solution in figure 15 2. careful examination shows that a small supercooling “dip” occurred in both curves. it is also apparent that the. tf = 6.52 c. tf = 3.35 c. figure 15 1. cooling curve of a pure liquid. figure 15 2. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is.
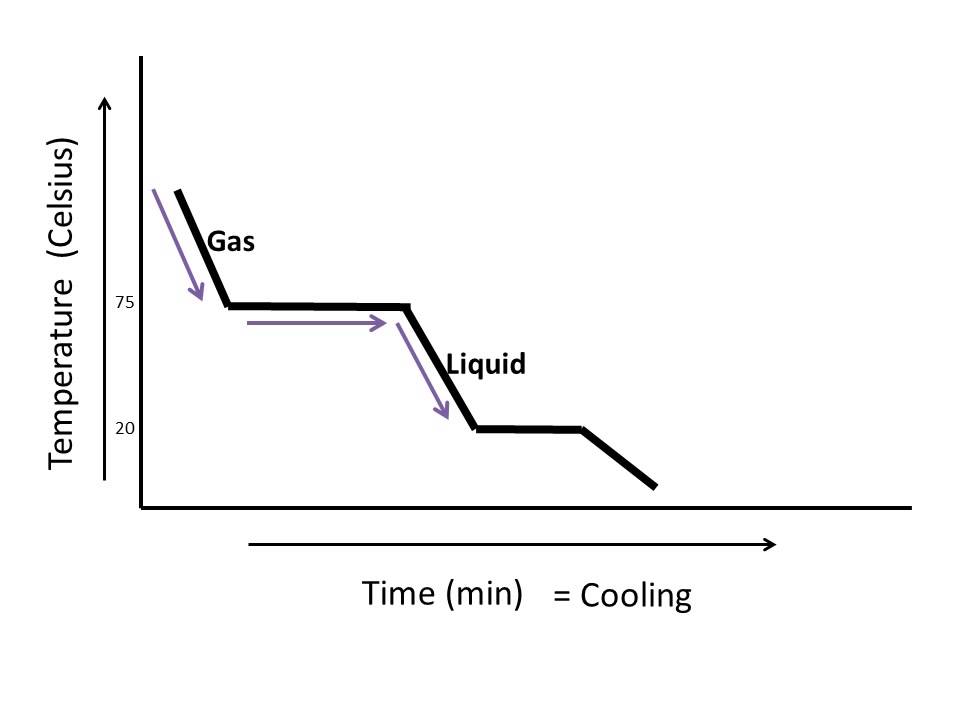
How To Read A Cooling Curve Youtube An experimental cooling curve for a pure liquid is shown in figure 15 1 and for a solution in figure 15 2. careful examination shows that a small supercooling “dip” occurred in both curves. it is also apparent that the. tf = 6.52 c. tf = 3.35 c. figure 15 1. cooling curve of a pure liquid. figure 15 2. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is.
Solved What Happens In The Cooling Curve Of A Liquid When The
Solved The Plateau Portion Of The Cooling Curve Is Characteristic For

Comments are closed.