Dot And Cross Diagrams For Simple Covalent Molecules Of Oxygen O2
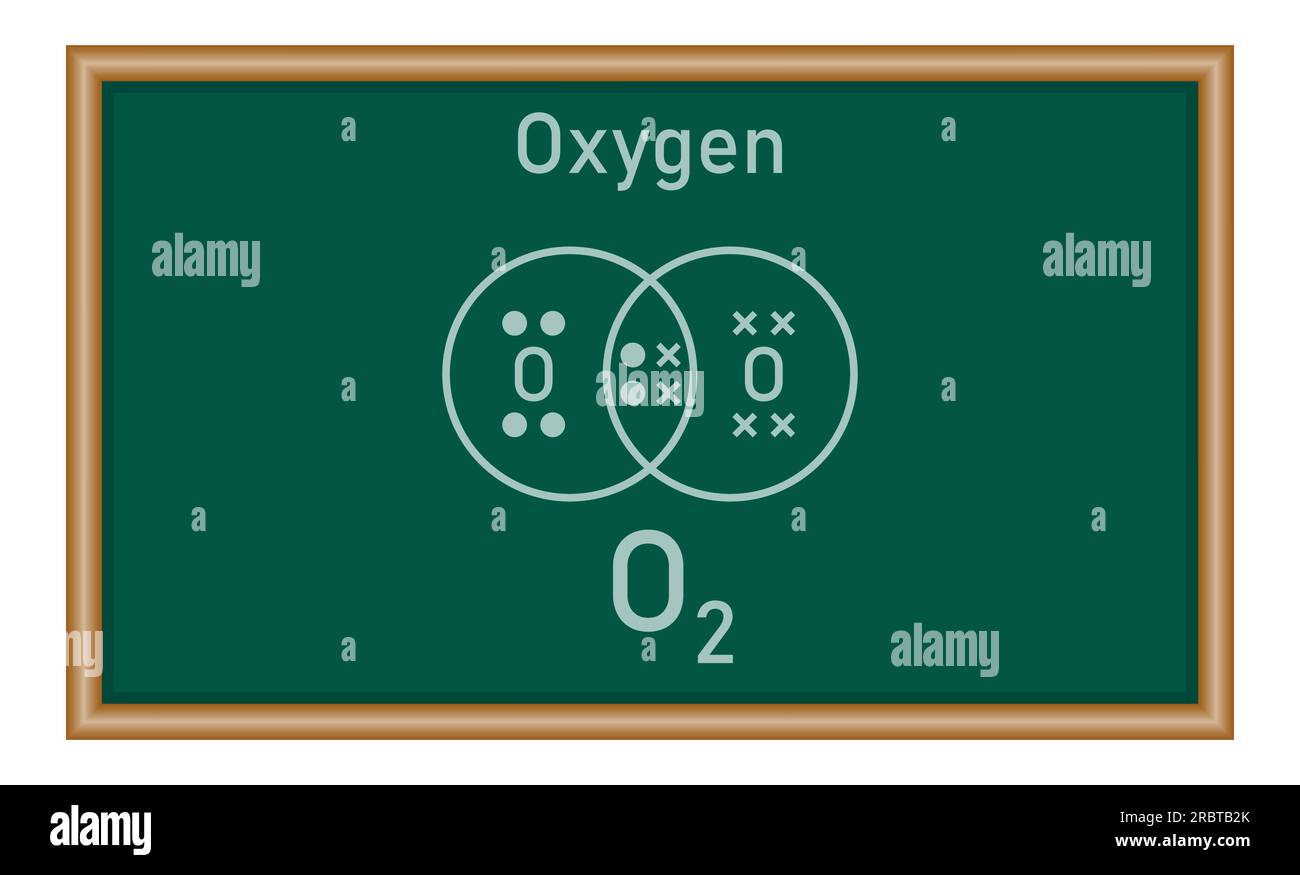
Dot And Cross Diagrams For Simple Covalent Molecules Of Oxygen O2 We can use dot and cross diagrams to show how a pair of electrons forms a covalent bond. here is the dot and cross diagram for oxygen (o 2 ), a diatomic molecule. An electron deficient atom is an atom that has an unfilled outer orbital. so both electrons are from the same atom. this type of bonding is called dative covalent bonding or coordinate bonding. an example with a dative bond is in an ammonium ion. the hydrogen ion, h is electron deficient and has space for two electrons in its shell.
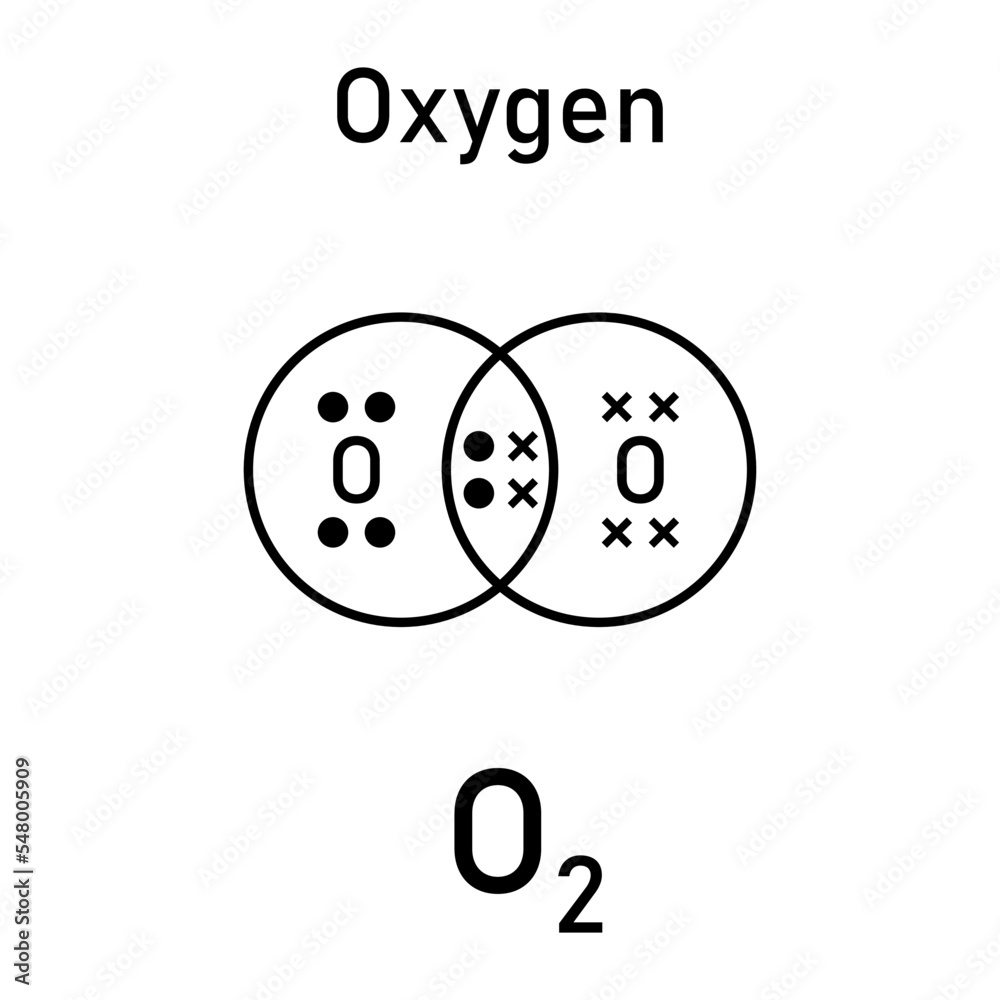
Dot And Cross Diagrams For Simple Covalent Molecules Of Oxygen O2 Dot and cross diagrams for covalent compounds. covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co 2. these small molecules are known as simple molecules. small covalent molecules can be represented by dot and cross diagrams. you need to be able to describe and draw the structures of the molecules below:. The formula is h 2 o so the dot and cross diagram includes two h atoms and one o atom. h has 1 outer electron. o has 6 outer electrons. the h circles must each overlap the o circle. nitrogen. The dot and cross diagram is a useful tool in chemistry to represent the bonding between atoms in a molecule. it visualizes the sharing or transfer of electrons between atoms and helps in understanding the overall structure of the compound. in the case of oxygen, which has the atomic symbol o and atomic number 8, it forms strong double covalent. Modelling molecules; drawing dot and cross diagrams; oxygen, o: electrons: 6: covalent bonds: 2: to work out how many circles to draw for a simple molecular close molecular refers to a.
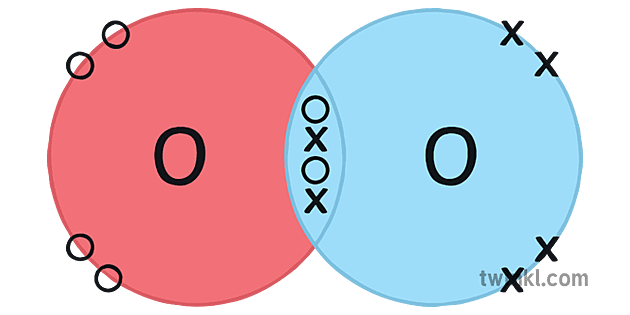
O2 Oksijeni Covalent Bonding Dot Cross Diagram Science Ks4 Illustration The dot and cross diagram is a useful tool in chemistry to represent the bonding between atoms in a molecule. it visualizes the sharing or transfer of electrons between atoms and helps in understanding the overall structure of the compound. in the case of oxygen, which has the atomic symbol o and atomic number 8, it forms strong double covalent. Modelling molecules; drawing dot and cross diagrams; oxygen, o: electrons: 6: covalent bonds: 2: to work out how many circles to draw for a simple molecular close molecular refers to a. Draw dot and cross diagrams for simple covalent molecules 2 identify single, double and triple covalent bonds introduction a covalent bond is a shared pair of electrons. we use dot and cross diagrams to show which atom the electrons in a covalent bond come from. each atom usually makes enough covalent bonds to fill its outer shell. instructions. #tutorials #chemistry #lessonsin this video, we will draw dot and cross diagram of oxygen molecule. from there, we will determine the structural formula of o.

Comments are closed.