Electrochemistry Electrochemistry Chemistry Lessons Chemistry
Chemistry Electrochemistry 21 0 Electrochemistry 25 Lessons Supplemental modules (analytical chemistry) electrochemistry. is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. electrochemistry is the study of electricity and how it relates to chemical reactions. in electrochemistry, electricity can be generated by movements of electrons from one element to. Electrochemistry basics. electrochemistry is the study of chemical processes that cause electrons to move. this movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation reduction ("redox") reaction.

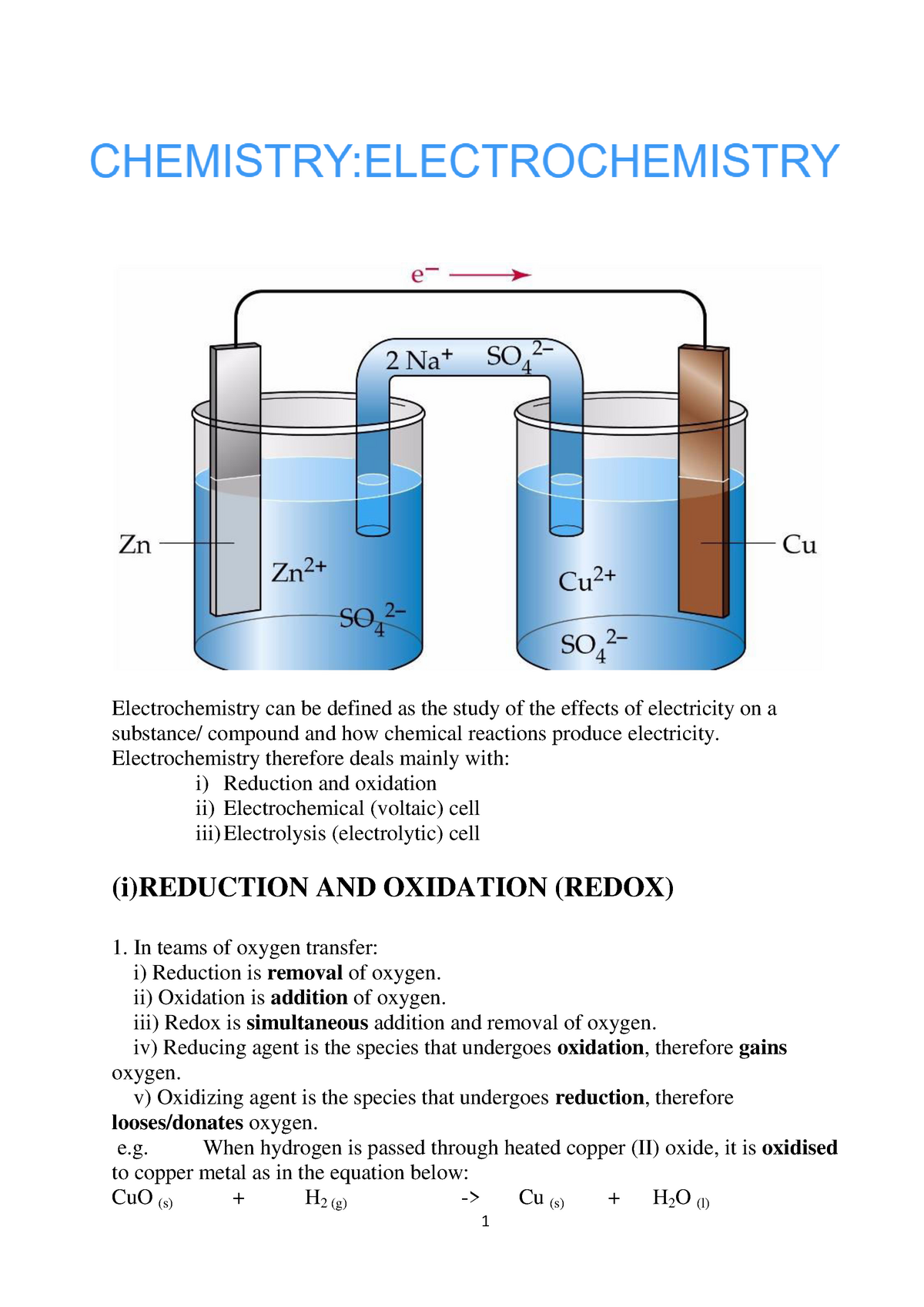
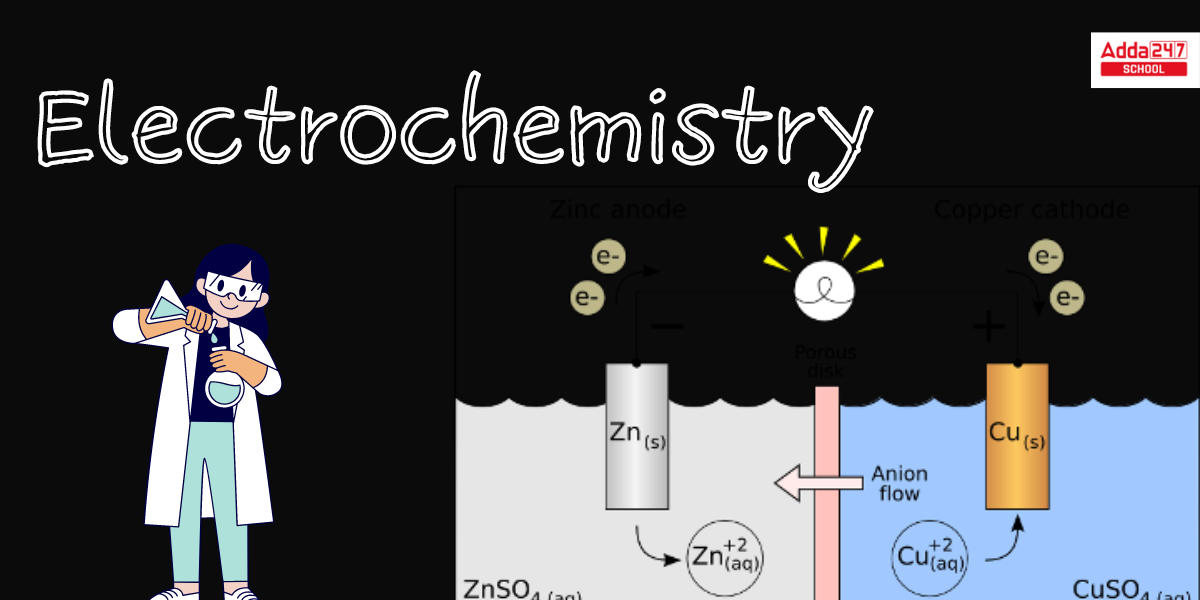
Chemistry 30 Electrochemistry Lab Electrochemical Cells Lesson 14: electrochemistry. electrochemistry questions. electrochemistry. redox reaction from dissolving zinc in copper sulfate. introduction to galvanic voltaic cells. electrodes and voltage of galvanic cell. shorthand notation for galvanic voltaic cells. free energy and cell potential. standard reduction potentials. 21675. in oxidation–reduction (redox) reactions, electrons are transferred from one species (the reductant) to another (the oxidant). this transfer of electrons provides a means for converting chemical energy to electrical energy or vice versa. the study of the relationship between electricity and chemical reactions is called electrochemistry. To understand electrochemistry, you will combine the concepts of gibbs free energy, electron flow, and chemical transformation. in this course, you will explore key concepts of acid base reactions and their relation to chemical equilibrium. you will learn the significance of electrochemistry, understanding how electrical, chemical, and. An electrochemical cell is something that changes chemical energy to electrical energy. it contains two compartments, each with an electrode submerged in an electrolyte. the electrode is just a.

Electrochemistry Featuring Electrolysis And Fuel Cells Mrs Elliott To understand electrochemistry, you will combine the concepts of gibbs free energy, electron flow, and chemical transformation. in this course, you will explore key concepts of acid base reactions and their relation to chemical equilibrium. you will learn the significance of electrochemistry, understanding how electrical, chemical, and. An electrochemical cell is something that changes chemical energy to electrical energy. it contains two compartments, each with an electrode submerged in an electrolyte. the electrode is just a. Electrochemistry is the combined study of chemical reactions and electricity. there are two ways chemical reactions and electricity can interact. electricity can be used to force certain chemical reactions to happen, or certain chemical reactions can be used to generate electricity. it is important to define electricity here. Chemistry raised to the power of awesome! that’s what this video talks about with electrochemistry. contained within, we discuss electrochemical reactions, half reactions, how batteries work, galvanic cells, voltage, standard reduction potential, cell potential, electrolysis, and electro plating and the things that go into making it possible.
Electrochemistry Meaning Electrochemical Cells Notes Pdf For Neet Electrochemistry is the combined study of chemical reactions and electricity. there are two ways chemical reactions and electricity can interact. electricity can be used to force certain chemical reactions to happen, or certain chemical reactions can be used to generate electricity. it is important to define electricity here. Chemistry raised to the power of awesome! that’s what this video talks about with electrochemistry. contained within, we discuss electrochemical reactions, half reactions, how batteries work, galvanic cells, voltage, standard reduction potential, cell potential, electrolysis, and electro plating and the things that go into making it possible.

Electrochemistry Chemistry Formula Physics Wallah Electrochemistry

Comments are closed.