Element Name And Symbol Atomic Number
/periodic-table-of-the-elements-2017--illustration-769723031-5ac10eb6a9d4f9003769784d.jpg)
Element List Atomic Number Element Name And Symbol The discoverer gets to suggest a new name and symbol, but the iupac has the final word. until a new name is approved, the systematic element name is used. this name describes the atomic number of the element, followed by the ium suffix. for example, element 120 has the temporary name of unbinilium. A chemical element, often simply called an element, is a type of atom which has a specific number of protons in its atomic nucleus (i.e., a specific atomic number, or z). [ 1 ] the definitive visualisation of all 118 elements is the periodic table of the elements , whose history along the principles of the periodic law was one of the founding developments of modern chemistry .
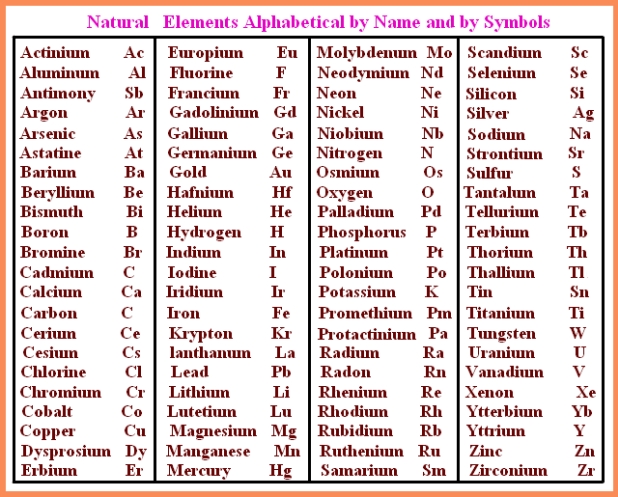
Periodic Table With Element Names Hd Periodic Table Timeline Most of the symbols are similar to the name of the element but some symbols of elements have latin roots. an example for this is silver which is denoted by ag from its latin name “argentum”. another such example would be the symbol ‘fe’ which is used to denote iron and can be traced to the latin word for iron, “ferrum”. This is a list of elements by atomic number with symbol. list of elements atomic number name symbol group period number 1 hydrogen h 1 1 2 helium he 18 1 3. Before a name and symbol are approved, an element may be referred to by its atomic number (e.g., element 120) or by its systematic element name. the systematic element name is a temporary name that is based on the atomic number as a root and the ium ending as a suffix. for example, hypothetical element 120 has the temporary name unbinilium. The number of protons in the nucleus is called the atomic number. the atomic number of each element is unique. the combined number of protons and neutrons in an atom is called the atomic mass number. while the atomic number always stays the same. some elements have atoms with different atomic mass numbers.

Comments are closed.