Fda Approves Fixed Dose Macitentan Tadalafil Tablets For Pah Daily Dose
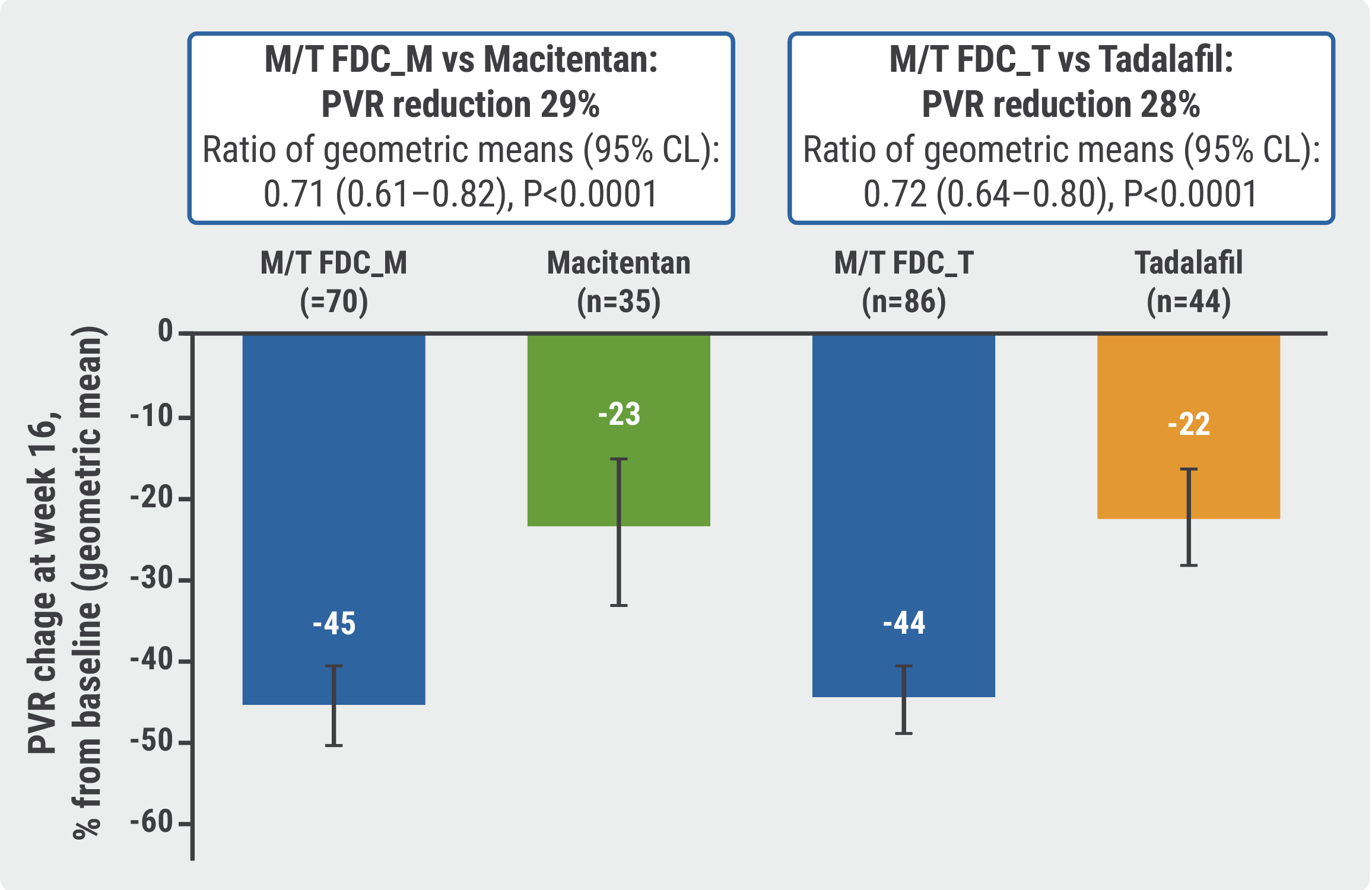
Fixed Dose Macitentan Plus Tadalafil Superior To Either Agent Alone In Raritan, nj, march 22, 2024 – johnson & johnson today announced that the u.s. food and drug administration (fda) has approved opsynvi ® – a single tablet combination of macitentan, an endothelin receptor antagonist (era), and tadalafil, a phosphodiesterase 5 (pde5) inhibitor – for the chronic treatment of adults with pulmonary arterial hypertension (pah, world health organization [who. The 2 agents are typically prescribed together as guideline directed initial therapy for pah. the foundation for the fda approval is the a due study, a double blind, randomized, active control parallel group study that compared the efficacy and safety of the fixed dose macitentan and tadalafil combination to each drug as monotherapy in adults.

Fixed Dose Macitentan Tadalafil Combo Gets Fda Approval For Pah The u.s. food and drug administration (fda) has approved once daily, fixed dose tablets containing a combination of macitentan and tadalafil — to be marketed under the brand name opsynvi — to treat adults with pulmonary arterial hypertension (pah). the approval marks the first single tablet treatment combination to become available to. The a due (clinical study to compare the efficacy and safety of macitentan and tadalafil monotherapies with the corresponding fixed dose combination therapy in subjects with pulmonary arterial hypertension) study investigated the efficacy and safety of a single tablet fdc of macitentan 10 mg and tadalafil 40 mg (m t fdc) as a once daily. The foundation for the fda approval is the a due study, a double blind, randomized, active control parallel group study that compared the efficacy and safety of the fixed dose macitentan and tadalafil combination to each drug as monotherapy in adults with pah (who fc ii or iii) who were treatment naïve or on stable doses of an era or pde5. Pah is a rare, progressive blood vessel disorder characterized by the constriction of small pulmonary arteries and elevated blood pressure in the pulmonary circulation that eventually leads to right heart failure. opsynvi contains macitentan, an endothelin receptor antagonist (era), and tadalafil, a phosphodiesterase 5 (pde5) inhibitor.

The Us Food And Drug Administration Fda Approves The Single Tablet The foundation for the fda approval is the a due study, a double blind, randomized, active control parallel group study that compared the efficacy and safety of the fixed dose macitentan and tadalafil combination to each drug as monotherapy in adults with pah (who fc ii or iii) who were treatment naïve or on stable doses of an era or pde5. Pah is a rare, progressive blood vessel disorder characterized by the constriction of small pulmonary arteries and elevated blood pressure in the pulmonary circulation that eventually leads to right heart failure. opsynvi contains macitentan, an endothelin receptor antagonist (era), and tadalafil, a phosphodiesterase 5 (pde5) inhibitor. The fixed dose combination of macitentan tadalafil is now indicated for the chronic treatment of pah in people who are treatment naive or who are already on an endothelin receptor antagonist like. Opsynvi® combines two proven treatments with established efficacy and safety profiles into one tablet to be taken once daily, offering an option that helps to support the implementation of clinical guideline recommendations for early use of combination therapy.1 the comprehensive pah portfolio at johnson & johnson now includes treatments that address all three foundational and guideline.
Single Tablet Combo Of Macitentan And Tadalafil Receives Us Fda The fixed dose combination of macitentan tadalafil is now indicated for the chronic treatment of pah in people who are treatment naive or who are already on an endothelin receptor antagonist like. Opsynvi® combines two proven treatments with established efficacy and safety profiles into one tablet to be taken once daily, offering an option that helps to support the implementation of clinical guideline recommendations for early use of combination therapy.1 the comprehensive pah portfolio at johnson & johnson now includes treatments that address all three foundational and guideline.

Opsynvi Macitentan And Tadalafil Becomes The First And Only Health

Fda Approves Fixed Dose Macitentan Tadalafil Tablets For Pah Daily Dose

Comments are closed.