First Order Reaction Rate Calculation Example Vrogue Co

First Order Reaction Definition Examples And Equations The differential equation describing first order kinetics is given below: rate = − d[a] dt = k[a]1 = k[a] the "rate" is the reaction rate (in units of molar time) and k is the reaction rate coefficient (in units of 1 time). however, the units of k vary for non first order reactions. these differential equations are separable, which simplifies. In a first order reaction, the reaction rate is directly proportional to the concentration of one of the reactants. first order reactions often have the general form a → products. the differential rate for a first order reaction is as follows: rate = −Δ[a] Δt = k[a] (14.5.1) (14.5.1) rate = − Δ [a] Δ t = k [a] if the concentration of.
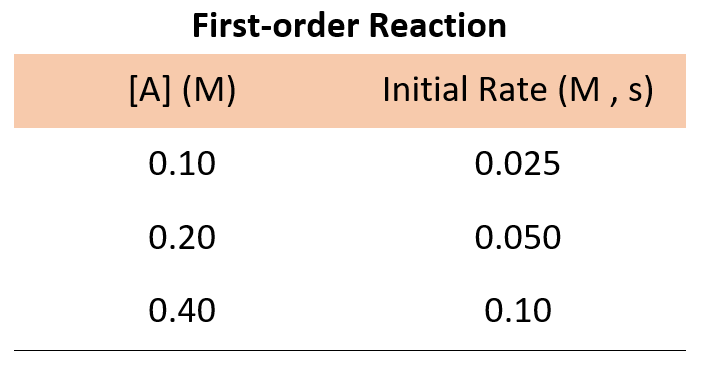
How To Determine The Reaction Order Chemistry Steps Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time. If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall. Rate equation. in chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an empirical differential mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction.

First Order Reaction Rate Law Definition Equation Examples Rate equation. in chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an empirical differential mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction. A first order reaction can be defined as a chemical reaction for which the reaction rate is entirely dependent on the concentration of only one reactant. in such reactions, if the concentration of the first order reactant is doubled, then the reaction rate is also doubled. similarly, if the first order reactant concentration is increased five. Lesson 2: relationship between reaction concentrations and time. first order reactions. first order reaction (with calculus) plotting data for a first order reaction. half life of a first order reaction. worked example: using the first order integrated rate law and half life equations. second order reactions.

Comments are closed.