First Order Reaction Units
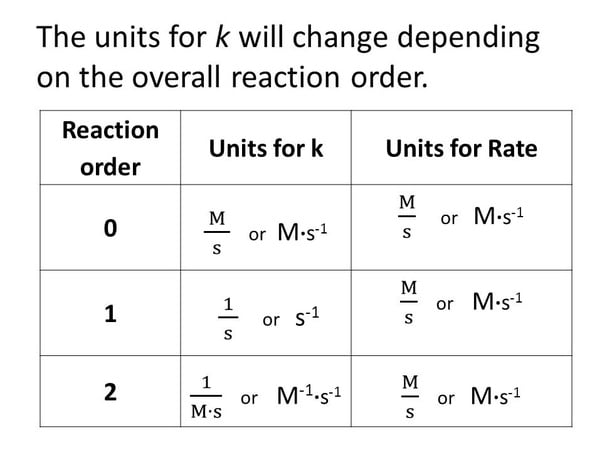
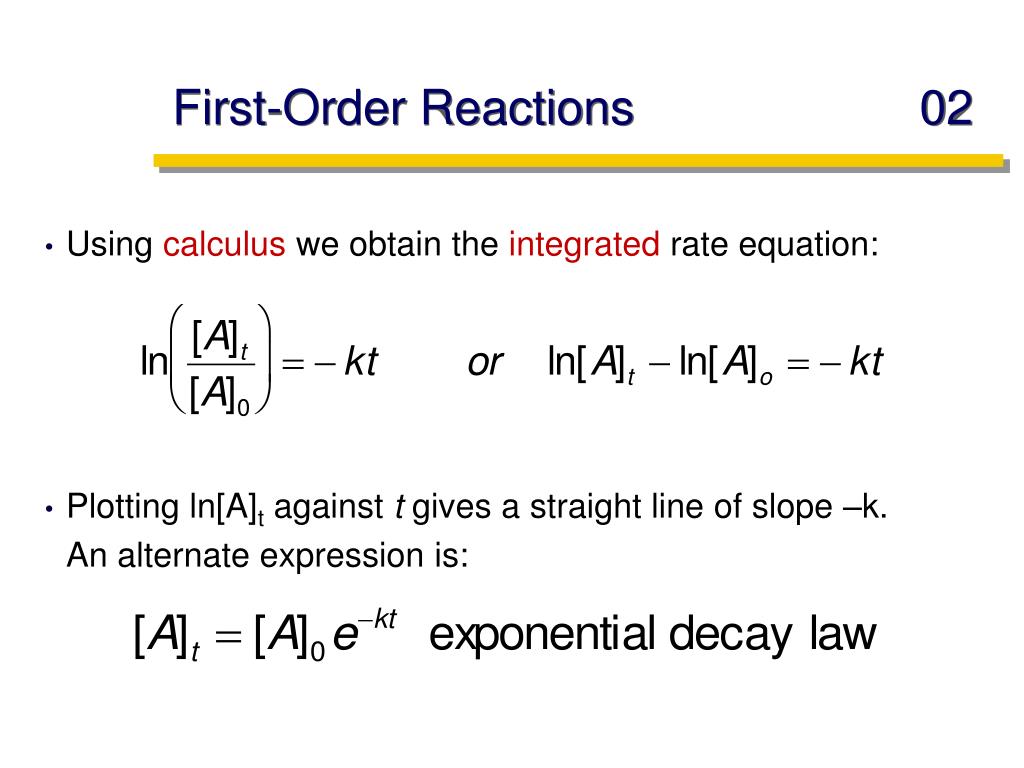
First Order Reaction Units The differential equation describing first order kinetics is given below: rate = − d[a] dt = k[a]1 = k[a] the "rate" is the reaction rate (in units of molar time) and k is the reaction rate coefficient (in units of 1 time). however, the units of k vary for non first order reactions. these differential equations are separable, which simplifies. In a first order reaction, the reaction rate is directly proportional to the concentration of one of the reactants. first order reactions often have the general form a → products. the differential rate for a first order reaction is as follows: rate = −Δ[a] Δt = k[a] (14.5.1) (14.5.1) rate = − Δ [a] Δ t = k [a] if the concentration of.
First Order Reaction Units From this equation, a general formula for the units of k is obtained which is: k units = m1 n · t 1. where n is the reaction order. for example, let’s say we want to determine the units of the rate constant for third order reactions. n = 3, and therefore, k units = m1 3 · t 1 = m 2 · t 1. if the time is seconds, then the units will be:. Aboutabout this video. transcript. the units of the rate constant, k, depend on the overall reaction order. the units of k for a zero order reaction are m s, the units of k for a first order reaction are 1 s, and the units of k for a second order reaction are 1 (m·s). created by yuki jung. Learn what a first order reaction is, how to identify it, and how to write its rate laws. find examples, graphs, and half life of first order reactions. Learn what a first order reaction is, how to determine its rate law and half life, and see examples of common first order reactions. a first order reaction is a chemical reaction where the reaction rate depends linearly on the reactant’s concentration.
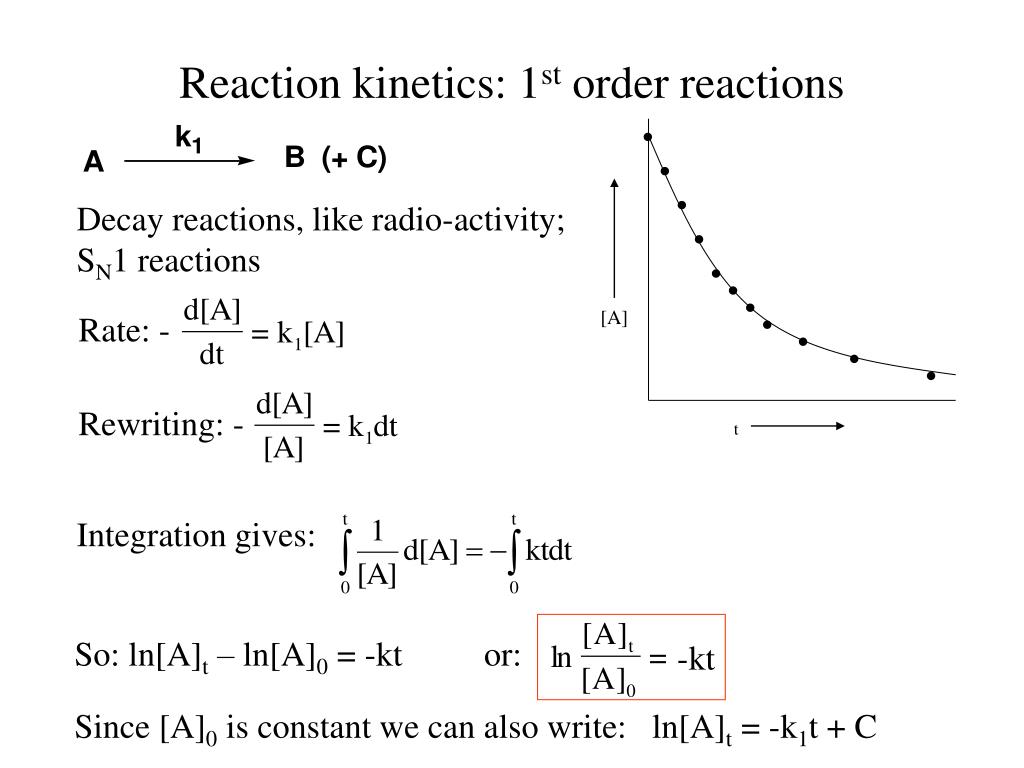
Ppt Reaction Kinetics 1 St Order Reactions Powerpoint Presentation Learn what a first order reaction is, how to identify it, and how to write its rate laws. find examples, graphs, and half life of first order reactions. Learn what a first order reaction is, how to determine its rate law and half life, and see examples of common first order reactions. a first order reaction is a chemical reaction where the reaction rate depends linearly on the reactant’s concentration. Learn how to identify and apply first order reactions, where the rate is proportional to the concentration of one reactant. find out the units of rate constant, the integrated rate law, and the half life of first order reactions. Learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant. find out what first order and second order reactions are, and how to calculate them using kinetic data.

Zeroth Order Reaction Learn how to identify and apply first order reactions, where the rate is proportional to the concentration of one reactant. find out the units of rate constant, the integrated rate law, and the half life of first order reactions. Learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant. find out what first order and second order reactions are, and how to calculate them using kinetic data.

First Order Reaction Units

Comments are closed.