First Order Reaction Units Vrogue Co

First Order Reaction Rate Calculation Example Vrogue Co Thus the reaction is first order. knowing this, we can calculate the rate constant using the differential rate law for a first order reaction and the data in any row of table 14.5.1. for example, substituting the values for experiment 3 into equation 14.5.1, 3.6 × 10 −5 m min = k (0.024 m) 1.5 × 10 −3 min −1 = k. Because the units of the reaction rate are always moles per liter per second, the units of a first order rate constant are reciprocal seconds (s −1). the integrated rate law for a first order reaction can be written in two different ways: one using exponents and one using logarithms.
First Order Reaction Units Vrogue Co Thus, the equation of a straight line is applicable: ln[a] = −kt ln[a]o. (2.3.2) (2.3.2) ln [a] = − k t ln [a] o. to test if it the reaction is a first order reaction, plot the natural logarithm of a reactant concentration versus time and see whether the graph is linear. if the graph is linear and has a negative slope, the reaction must. From this equation, a general formula for the units of k is obtained which is: k units = m1 n · t 1. where n is the reaction order. for example, let’s say we want to determine the units of the rate constant for third order reactions. n = 3, and therefore, k units = m1 3 · t 1 = m 2 · t 1. if the time is seconds, then the units will be:. A first order reaction depends on the concentration of only one reactant (a unimolecular reaction). other reactants can be present, but their concentration has no effect on the rate. the rate law for a first order reaction is [] = [], the unit of k is s 1. [18]. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time.
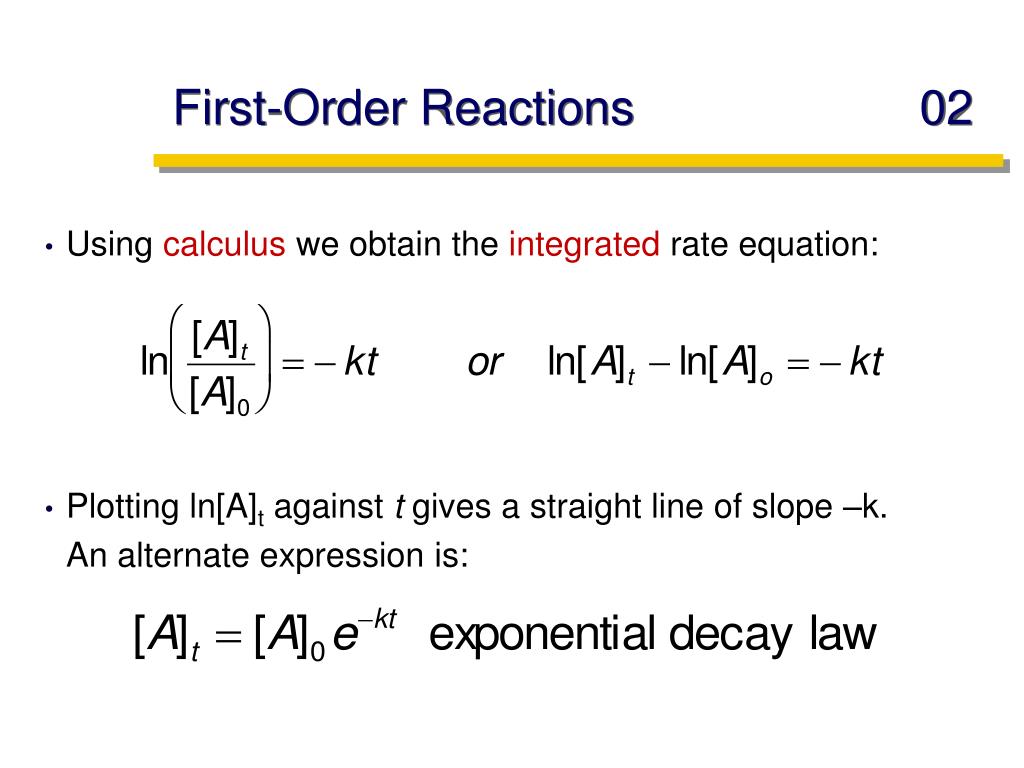
First Order Reaction Units Vrogue Co A first order reaction depends on the concentration of only one reactant (a unimolecular reaction). other reactants can be present, but their concentration has no effect on the rate. the rate law for a first order reaction is [] = [], the unit of k is s 1. [18]. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time. The units for a rate constant will vary as appropriate to accommodate the overall order of the reaction. the unit of the rate constant for the second order reaction described in example 12.4 was determined to be l mol −1 s −1. l mol −1 s −1. for the third order reaction described in example 12.5, the unit for k was derived to be l 2 mol. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction.
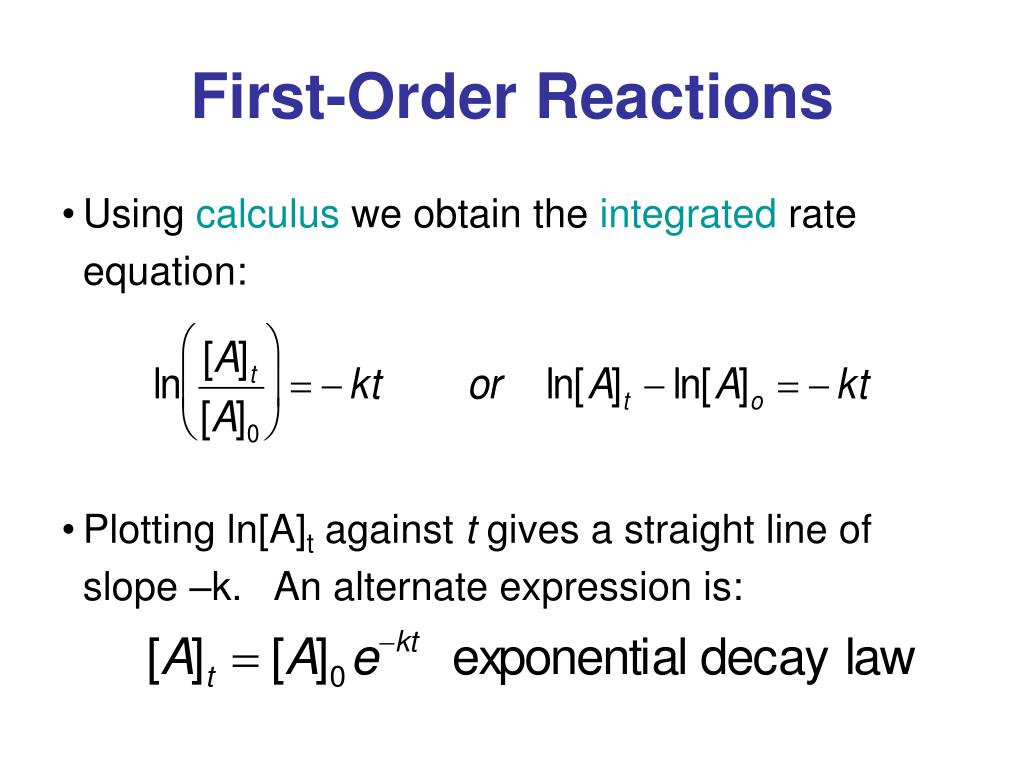
First Order Reaction Units Vrogue Co The units for a rate constant will vary as appropriate to accommodate the overall order of the reaction. the unit of the rate constant for the second order reaction described in example 12.4 was determined to be l mol −1 s −1. l mol −1 s −1. for the third order reaction described in example 12.5, the unit for k was derived to be l 2 mol. That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction.

First Order Reaction Units Vrogue Co

Comments are closed.