First Order Reactions Chemistry Steps
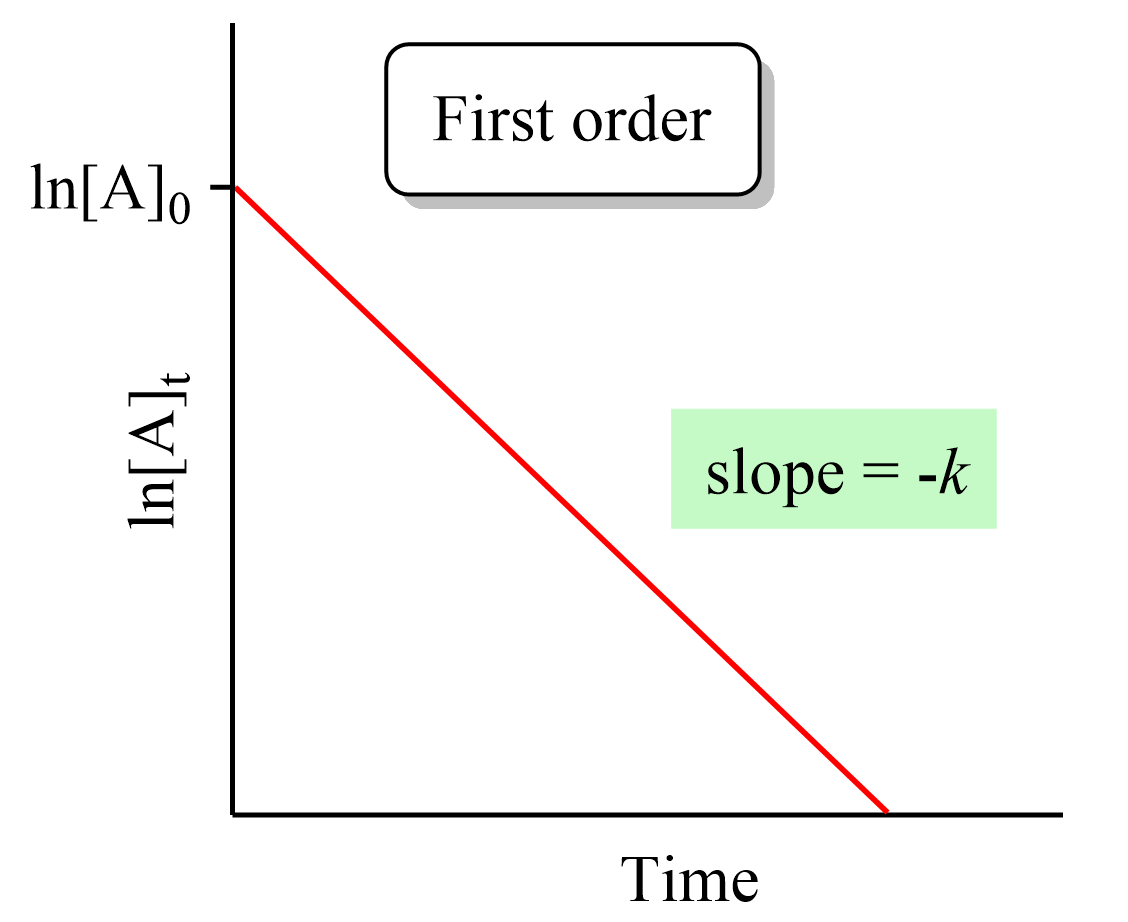
First Order Reactions Chemistry Steps In first order reactions, the rate of the reaction is directly linearly proportional to the concentration of the reactant. this can be seen in the differential rate law which shows how the rate of a reaction depends on the concentration of the reactant (s): a → products. rate = k[a]1. where k is the rate constant, and the exponent 1 is the. The differential equation describing first order kinetics is given below: rate = − d[a] dt = k[a]1 = k[a] the "rate" is the reaction rate (in units of molar time) and k is the reaction rate coefficient (in units of 1 time). however, the units of k vary for non first order reactions. these differential equations are separable, which simplifies.
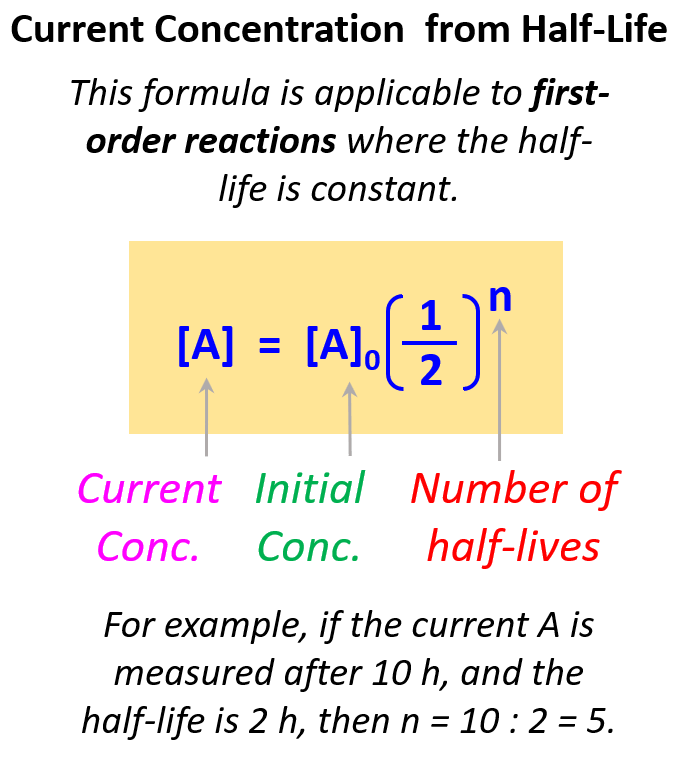
First Order Reactions Chemistry Steps A → products. rate = k[a]n. where k is the rate constant and n is the reaction order. our objective is to determine the reaction order by calculating the n from a set of experiments. keep in mind that: if n = 0, the reaction is zero order, and the rate is independent of the concentration of a. if n = 1, the reaction is first order, and the. In a first order reaction, the reaction rate is directly proportional to the concentration of one of the reactants. first order reactions often have the general form a → products. the differential rate for a first order reaction is as follows: rate = −Δ[a] Δt = k[a] (14.5.1) (14.5.1) rate = − Δ [a] Δ t = k [a] if the concentration of. Given the rate law equation: rate = k[a]1[b]2. 2. determine: a) the reaction order with respect to a, b) the reaction order with respect to b, and c) the total reaction order for the equation. 3. assuming the reaction occurs in one elementary step, propose a chemical equation using p as the symbol for your product. A first order reaction can be defined as a chemical reaction for which the reaction rate is entirely dependent on the concentration of only one reactant. in such reactions, if the concentration of the first order reactant is doubled, then the reaction rate is also doubled. similarly, if the first order reactant concentration is increased five.
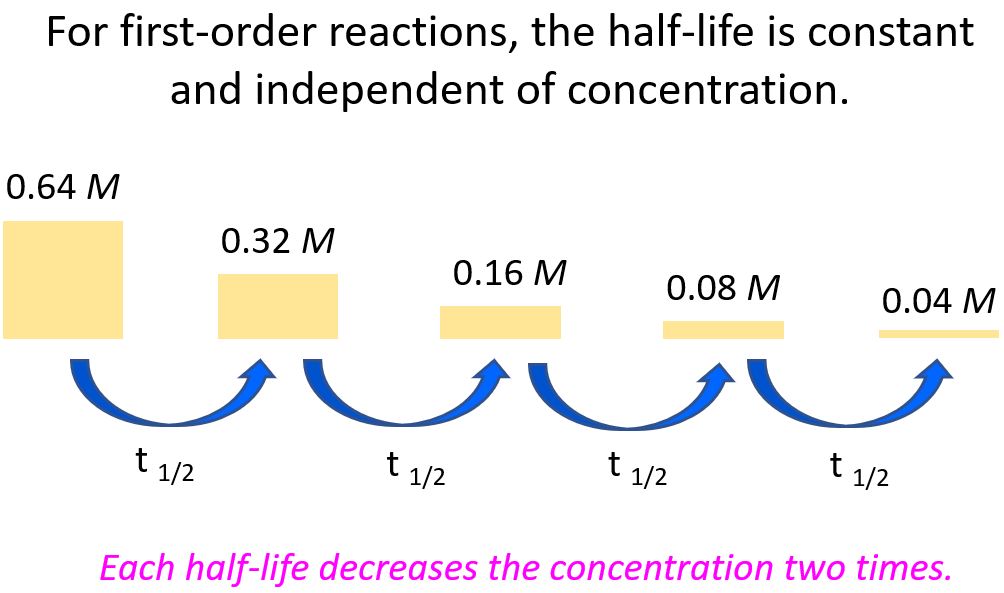
First Order Reactions Chemistry Steps Given the rate law equation: rate = k[a]1[b]2. 2. determine: a) the reaction order with respect to a, b) the reaction order with respect to b, and c) the total reaction order for the equation. 3. assuming the reaction occurs in one elementary step, propose a chemical equation using p as the symbol for your product. A first order reaction can be defined as a chemical reaction for which the reaction rate is entirely dependent on the concentration of only one reactant. in such reactions, if the concentration of the first order reactant is doubled, then the reaction rate is also doubled. similarly, if the first order reactant concentration is increased five. Mechanism 2. remember that in simple cases, where the slow step is the first step of the mechanism, the orders tell you what is taking part in the slow step. in this case, the reaction is first order with respect to both a and b, so one molecule of each must be taking part in the slow step. that means that mechanism 2 is possible. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time.

First Order Reaction Definition Examples And Equations Mechanism 2. remember that in simple cases, where the slow step is the first step of the mechanism, the orders tell you what is taking part in the slow step. in this case, the reaction is first order with respect to both a and b, so one molecule of each must be taking part in the slow step. that means that mechanism 2 is possible. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time.
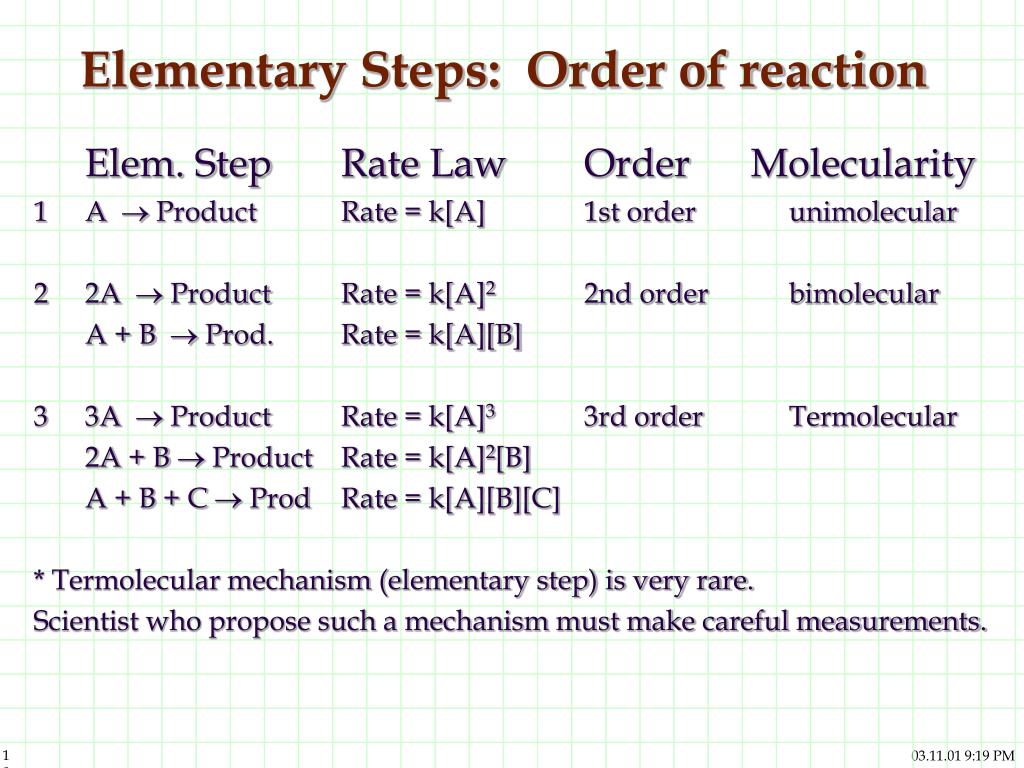
Ppt Reaction Mechanisms Steps Of A Reaction Powerpoint Presentation

Comments are closed.