Fixed Dose Macitentan Tadalafil Combo Gets Fda Approval For Pah

Fixed Dose Macitentan Tadalafil Combo Gets Fda Approval For Pah Raritan, nj, march 22, 2024 – johnson & johnson today announced that the u.s. food and drug administration (fda) has approved opsynvi ® – a single tablet combination of macitentan, an endothelin receptor antagonist (era), and tadalafil, a phosphodiesterase 5 (pde5) inhibitor – for the chronic treatment of adults with pulmonary arterial hypertension (pah, world health organization [who. The u.s. food and drug administration (fda) has approved once daily, fixed dose tablets containing a combination of macitentan and tadalafil — to be marketed under the brand name opsynvi — to treat adults with pulmonary arterial hypertension (pah). the approval marks the first single tablet treatment combination to become available to.

Janssen S Opsynvi Macitentan And Tadalafil Becomes The First And Only The a due (clinical study to compare the efficacy and safety of macitentan and tadalafil monotherapies with the corresponding fixed dose combination therapy in subjects with pulmonary arterial hypertension) study investigated the efficacy and safety of a single tablet fdc of macitentan 10 mg and tadalafil 40 mg (m t fdc) as a once daily. The fixed dose combination of macitentan tadalafil is now indicated for the chronic treatment of pah in people who are treatment naive or who are already on an endothelin receptor antagonist like. A fixed dose combination of the era macitentan and pde5i tadalafil (m t fdc) in a once daily, single tablet would simplify treatment. objectives the multicenter, double blind, adaptive phase 3 a due study investigated the efficacy and safety of m t fdc vs macitentan 10 mg and vs tadalafil 40 mg monotherapies in pah patients, including treatment. — both macitentan and tadalafil are available as single ingredients for pah. — opsynvi is the first fixed dose combination containing an era and pde5 inhibitor. • the efficacy of opsynvi was established in a double blind, adaptive, randomized, active controlled study in 187 patients with pah (who fc ii–iii).
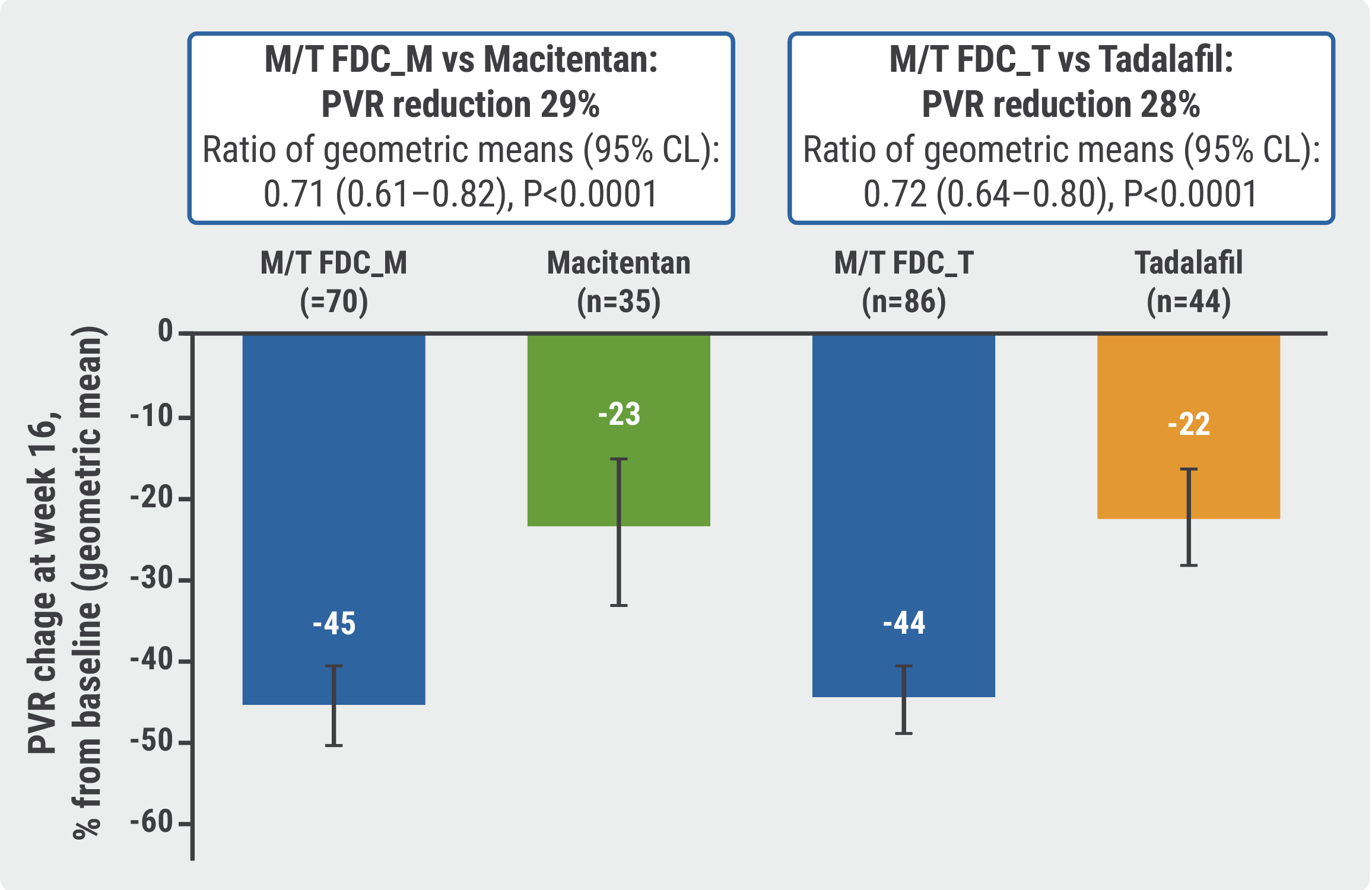
Fixed Dose Macitentan Plus Tadalafil Superior To Either Agent Alone In A fixed dose combination of the era macitentan and pde5i tadalafil (m t fdc) in a once daily, single tablet would simplify treatment. objectives the multicenter, double blind, adaptive phase 3 a due study investigated the efficacy and safety of m t fdc vs macitentan 10 mg and vs tadalafil 40 mg monotherapies in pah patients, including treatment. — both macitentan and tadalafil are available as single ingredients for pah. — opsynvi is the first fixed dose combination containing an era and pde5 inhibitor. • the efficacy of opsynvi was established in a double blind, adaptive, randomized, active controlled study in 187 patients with pah (who fc ii–iii). The foundation for the fda approval is the a due study, a double blind, randomized, active control parallel group study that compared the efficacy and safety of the fixed dose macitentan and tadalafil combination to each drug as monotherapy in adults with pah (who fc ii or iii) who were treatment naïve or on stable doses of an era or pde5. Contribution to literature: highlighted text has been updated as of january 23, 2024. the a due trial showed that fixed dose combination therapy with macitentan 10 mg daily tadalafil 40 mg daily is superior to either agent as monotherapy in reducing pvr at 6 weeks among patients with pah.

Macitentan Plus Tadalafil More Effective Than Monotherapy In Pah The foundation for the fda approval is the a due study, a double blind, randomized, active control parallel group study that compared the efficacy and safety of the fixed dose macitentan and tadalafil combination to each drug as monotherapy in adults with pah (who fc ii or iii) who were treatment naïve or on stable doses of an era or pde5. Contribution to literature: highlighted text has been updated as of january 23, 2024. the a due trial showed that fixed dose combination therapy with macitentan 10 mg daily tadalafil 40 mg daily is superior to either agent as monotherapy in reducing pvr at 6 weeks among patients with pah.

Fda Approves Fixed Dose Macitentan Tadalafil Tablets Opsynvi For
Single Tablet Combo Of Macitentan And Tadalafil Receives Us Fda

Comments are closed.