Gcse Chemistry Revision Covalent Bonding 1 Bonding In Hydrogen Chlorine And Hydrogen Chloride

Gcse Chemistry Revision Covalent Bonding 1 Bonding In Hydrogen Gcse workbooks amazon.co.uk dr shaun donnelly e b084fh9jpf?ref =dbs p pbk r00 abau 000000& encoding=utf8&tag=freesciencele 21&linkcode=ur2&linkid. Covalent bonds are strong – a lot of energy is needed to break them. substances with covalent bonds often form. molecules. with low melting and boiling points, such as hydrogen and water. these.
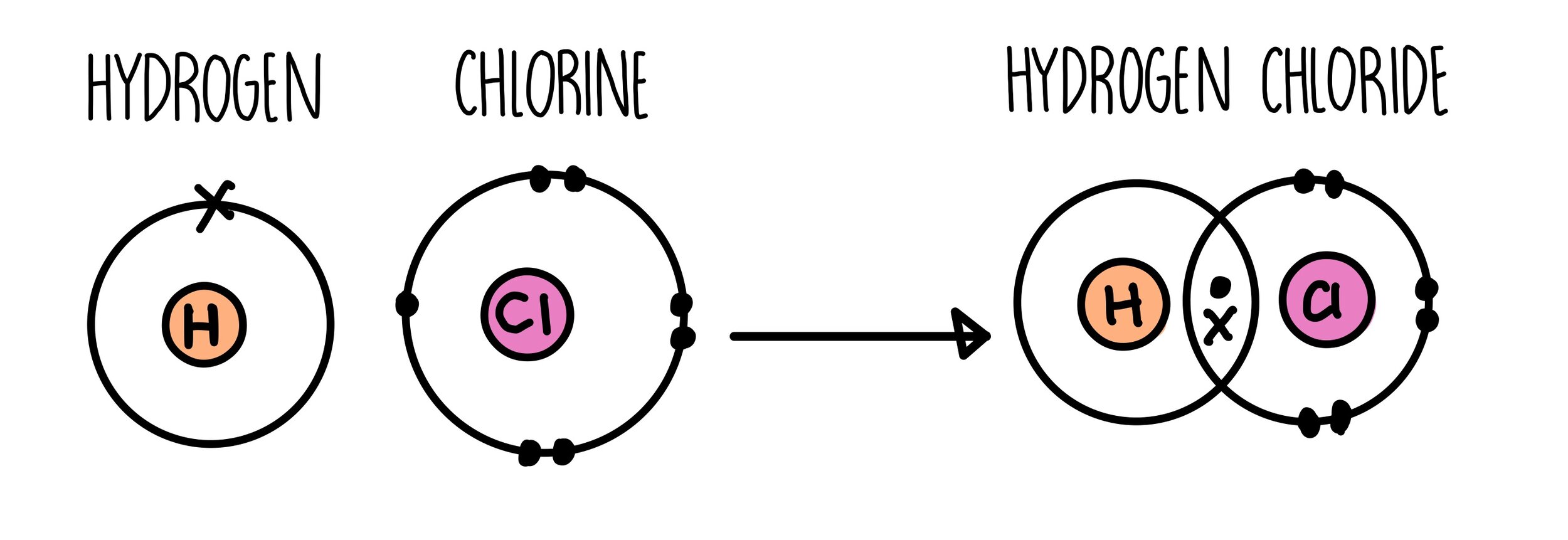
Covalent Bonding Gcse The Science Sauce A hydrogen atom has 1 electron in its outer shell. hydrogen can only form 1 bond. the hydrogen atom will share its 1 electron with chlorine to form one covalent bond and make a hydrogen chloride molecule (hcl). this is a picture of a hydrogen chloride molecule. by sharing the two electrons where the shells touch the hydrogen atom can count 2. Learn about and revise covalent bonds with this bbc bitesize gcse chemistry (ocr 21c) study guide. structures of a hydrogen atom and a chlorine atom., 1. a hydrogen atom with one electron and. The formation of covalent bonds. non metal atoms can share electrons with other non metal atoms to obtain a full outer shell of electrons. when atoms share pairs of electrons, they form covalent bonds. covalent bonds between atoms are very strong. when two or more atoms are chemically bonded together, they form ‘molecules’. Learn about and revise small molecules with this bbc bitesize gcse chemistry (aqa) study guide. how a covalent bond forms between a hydrogen atom and a chlorine atom, making hydrogen chloride.
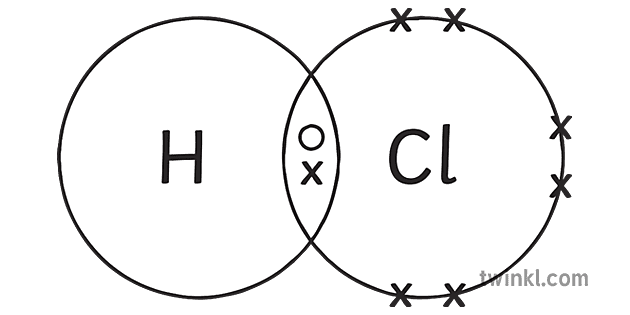
Hcl Hydrogen Chloride Covalent Bonding Dot Cross Diagram Science The formation of covalent bonds. non metal atoms can share electrons with other non metal atoms to obtain a full outer shell of electrons. when atoms share pairs of electrons, they form covalent bonds. covalent bonds between atoms are very strong. when two or more atoms are chemically bonded together, they form ‘molecules’. Learn about and revise small molecules with this bbc bitesize gcse chemistry (aqa) study guide. how a covalent bond forms between a hydrogen atom and a chlorine atom, making hydrogen chloride. These small molecules are known as simple molecules. small covalent molecules can be represented by dot and cross diagrams. you need to be able to describe and draw the structures of the following molecules using dot and cross diagrams: hydrogen (h 2), hydrogen chloride (hcl), water (h 2 o), methane (ch 4), oxygen (o 2) and carbon dioxide (co 2). Covalent bonds are a type of chemical bond in which two non metal atoms share one or more pairs of electrons. when two or more atoms bond in this way, they form a covalent molecule. these molecules can be made up of small individual units, or they can form giant, complex molecules. simple covalent molecules, such as water (h 2 o) or hydrogen.
Covalent Bonds Covalent Bonding Aqa Synergy Gcse Combined Science These small molecules are known as simple molecules. small covalent molecules can be represented by dot and cross diagrams. you need to be able to describe and draw the structures of the following molecules using dot and cross diagrams: hydrogen (h 2), hydrogen chloride (hcl), water (h 2 o), methane (ch 4), oxygen (o 2) and carbon dioxide (co 2). Covalent bonds are a type of chemical bond in which two non metal atoms share one or more pairs of electrons. when two or more atoms bond in this way, they form a covalent molecule. these molecules can be made up of small individual units, or they can form giant, complex molecules. simple covalent molecules, such as water (h 2 o) or hydrogen.

Covalent Bonding Igcse Chemistry Revision
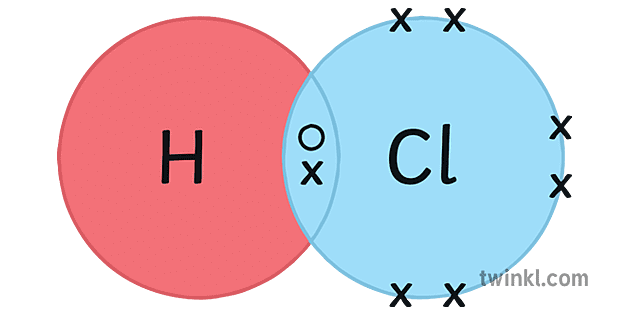
Hcl Hydrogen Chloride Covalent Bonding Dot Cross Diagram Science Sekondale

Comments are closed.