Heating And Cooling Curve Chart
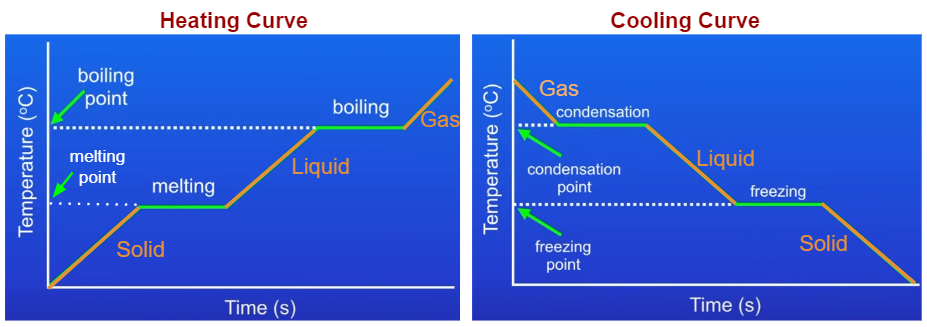
Heating And Cooling Graphs Examples Solutions Videos Notes Figure \(\pageindex{1}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. consider the example of heating a pot of water to boiling. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant.

Heating And Cooling Curves Read Chemistry Ck 12 Foundation Figure 2.5.3 2.5. 3: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. Heating cooling curves. a typical heating curve consists of a horizontal axis representing time and a vertical axis representing temperature. the curve is divided into distinct segments, each corresponding to a specific phase of the substance. during heating, the substance undergoes different phase transitions, such as solid to liquid (melting. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Heating And Cooling Curves For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. Heating and cooling curves. the experimental set up we imagined would generate a heating curve. heating and cooling curves are graphs. they plot a substance's temperature (y axis) against heat (x axis). for heating curves, we start with a solid and add heat energy. for cooling curves, we start with the gas phase and remove heat energy. Heating and cooling curves u 1. calculate the total amount of energy, h, required to cool a 34.5 g sample of ccl 4 from 88.5 oc to 45.0 oc. use the information below to draw a labeled cooling curve. c s = 0.287 𝑱 𝒈 ∙ °𝑪 melting point = 23oc c l = 0.866 𝑱 𝒈 ∙ °𝑪 boiling point = 77oc c g = 0.577 𝑱 𝒈 ∙ °𝑪 h fus.

Heating And Cooling Curves Overview Examples Expii Heating and cooling curves. the experimental set up we imagined would generate a heating curve. heating and cooling curves are graphs. they plot a substance's temperature (y axis) against heat (x axis). for heating curves, we start with a solid and add heat energy. for cooling curves, we start with the gas phase and remove heat energy. Heating and cooling curves u 1. calculate the total amount of energy, h, required to cool a 34.5 g sample of ccl 4 from 88.5 oc to 45.0 oc. use the information below to draw a labeled cooling curve. c s = 0.287 𝑱 𝒈 ∙ °𝑪 melting point = 23oc c l = 0.866 𝑱 𝒈 ∙ °𝑪 boiling point = 77oc c g = 0.577 𝑱 𝒈 ∙ °𝑪 h fus.

Heating And Cooling Curve Chart

Heating And Cooling Curve Introduction Plus Kinetic And Potential

Comments are closed.