Heating And Cooling Curve Explanation
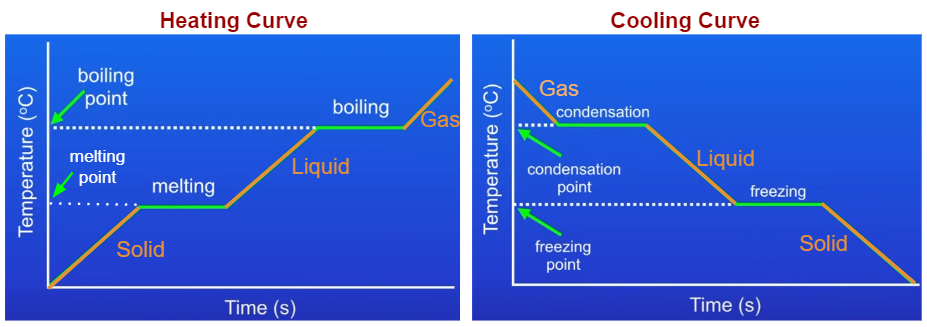
Heating And Cooling Graphs Examples Solutions Videos Notes The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The cooling curve and the heating curve are essentially the same curve but viewed in reverse. when studying a heating curve, we observe how a substance changes from solid to liquid, eventually to.

Heating And Cooling Curve Introduction Plus Kinetic And Potential The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Heating cooling curves. a typical heating curve consists of a horizontal axis representing time and a vertical axis representing temperature. the curve is divided into distinct segments, each corresponding to a specific phase of the substance. during heating, the substance undergoes different phase transitions, such as solid to liquid (melting. Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Heating And Cooling Curves Ck 12 Foundation Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have.

Heating And Cooling Curves Overview Examples Expii Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have.
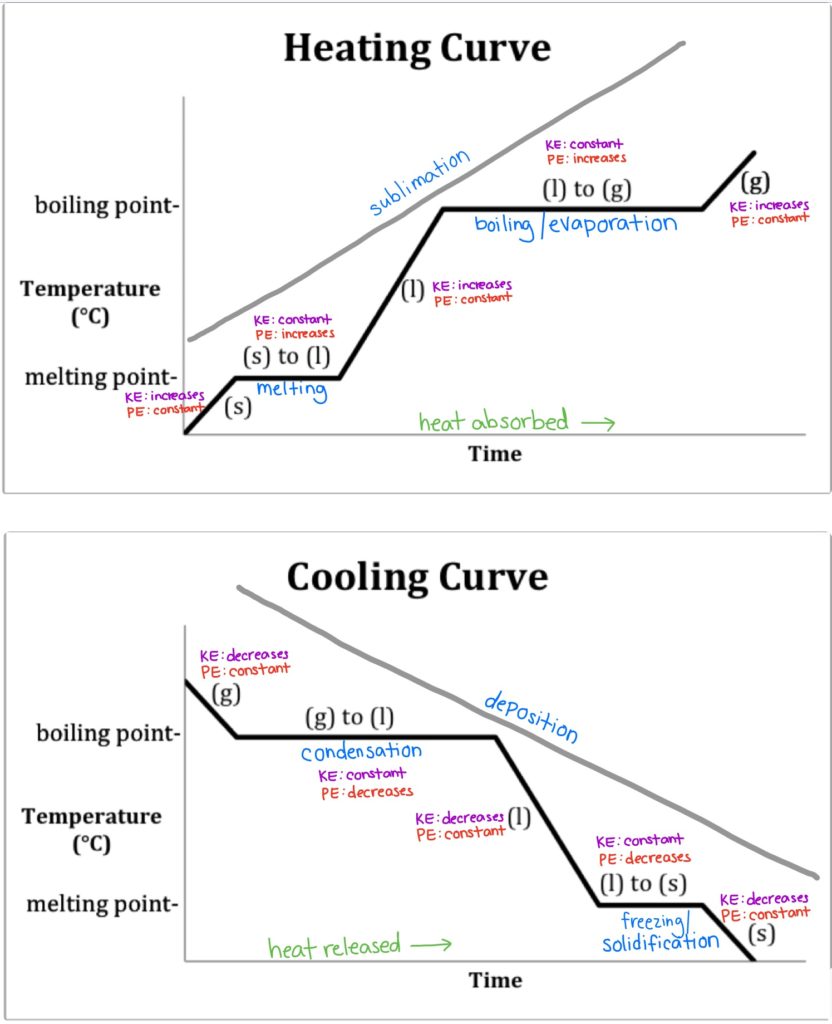
Heating And Cooling Curves

Heating Cooling Curves Definition Phases Examples Lesson

Comments are closed.