Heating And Cooling Curve Introduction Plus Kinetic And Potential

Heating And Cooling Curve Introduction Plus Kinetic And Potential An introduction to heating and cooling curve. in this video, i introduce heating and cooling curves and show the location of phase changes. " a typical heat. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have.
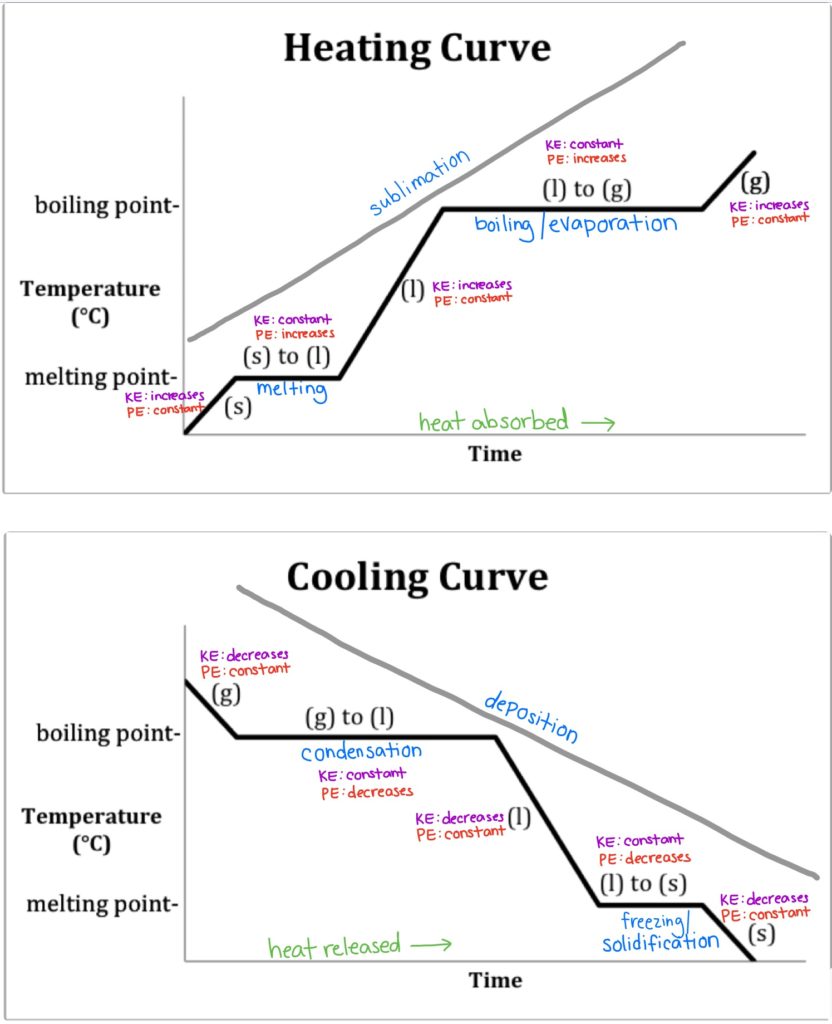
Kinetic And Potential Energy In Heating And Cooling Curves At William Figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. the change of state behavior of all substances can be represented with a heating curve of this type. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. Figure 2.5.3 2.5. 3: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. Heating and cooling curves. in the unit on thermochemistry, the relation between the amount of heat absorbed or related by a substance, q, and its accompanying temperature change, Δt, was introduced: q = mcΔt (3.7.0.1) (3.7.0.1) q = m c Δ t. where m is the mass of the substance and c is its specific heat. the relation applies to matter being.

Heating And Cooling Curves Overview Examples Expii Figure 2.5.3 2.5. 3: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. Heating and cooling curves. in the unit on thermochemistry, the relation between the amount of heat absorbed or related by a substance, q, and its accompanying temperature change, Δt, was introduced: q = mcΔt (3.7.0.1) (3.7.0.1) q = m c Δ t. where m is the mass of the substance and c is its specific heat. the relation applies to matter being. Heating and cooling curves have plateaus because they represent the phase changes of a substance, during which temperature remains constant despite the continuous input or removal of heat. this happens because the energy added or removed is used for breaking or forming intermolecular bonds rather than increasing the kinetic energy of the. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember.

Kinetic And Potential Energy In Heating And Cooling Curves At William Heating and cooling curves have plateaus because they represent the phase changes of a substance, during which temperature remains constant despite the continuous input or removal of heat. this happens because the energy added or removed is used for breaking or forming intermolecular bonds rather than increasing the kinetic energy of the. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember.

Comments are closed.