Heating Curve And Cooling Curve Of Water Enthalpy Of Fusion Vaporization

Heating Curve And Cooling Curve Of Water Enthalpy Of Fusion This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. as heat is added to water, the t. Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of.
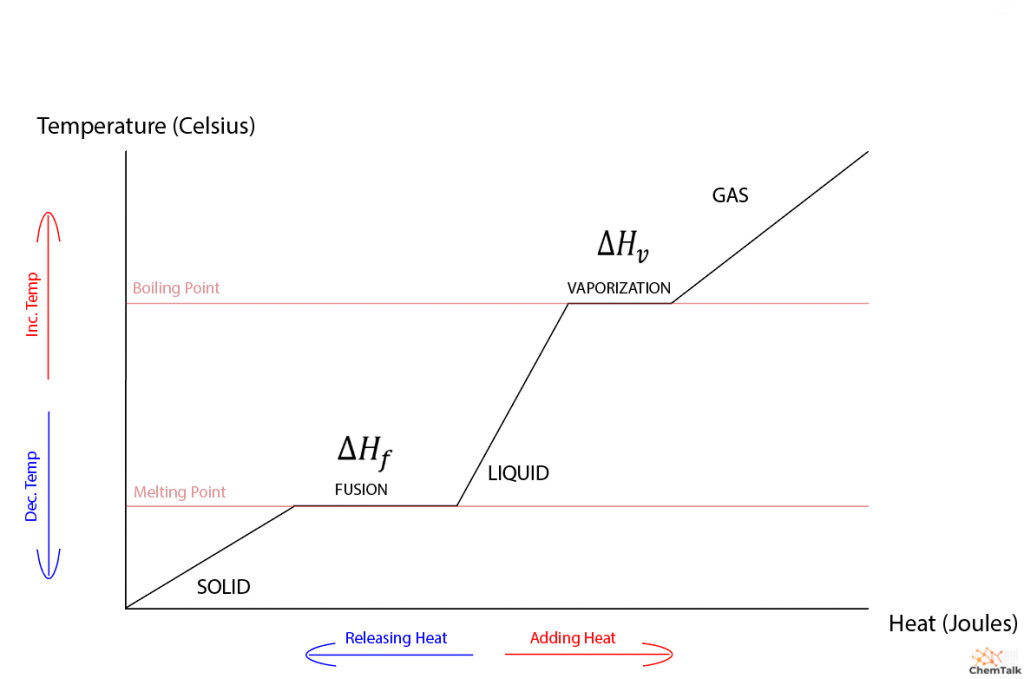
Heat Of Fusion Explained Chemtalk Figure 2.5.3 2.5. 3: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. Heating and cooling curves. general chemistry 13. liquids, solids & intermolecular forces heating and cooling curves. 13m. Heating curves. figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (c s) of ice. A heating curve graphically represents the phase transitions that a substance undergoes as heat is added to it. the plateaus on the curve mark the phase chan.

Heating Curve Of Water Enthalpy Of Fusion And Vaporization Youtube Heating curves. figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (c s) of ice. A heating curve graphically represents the phase transitions that a substance undergoes as heat is added to it. the plateaus on the curve mark the phase chan. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. when studying chemistry, “fusion” simply has the same definition as melting. in the classroom, you mostly use heat of fusion when a substance is at its melting point or. The heat required to convert all the liquid to a gas is called the enthalpy of vaporization, \(\delta h {vaporization}\). you can see the heat required to vaporize water is significantly more than the heat required to melt water. this is true for most substances. fusion involves simply breaking up the imf to allow for translational motion of.

Heating And Cooling Curves Ck 12 Foundation Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. when studying chemistry, “fusion” simply has the same definition as melting. in the classroom, you mostly use heat of fusion when a substance is at its melting point or. The heat required to convert all the liquid to a gas is called the enthalpy of vaporization, \(\delta h {vaporization}\). you can see the heat required to vaporize water is significantly more than the heat required to melt water. this is true for most substances. fusion involves simply breaking up the imf to allow for translational motion of.

Comments are closed.