How Are Cooling Curves Important For Phase Diagrams

Heating And Cooling Curves Overview Examples Expii A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa.

Ppt Phase Diagrams Introduction Powerpoint Presentation Free One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. Temperature–composition phase diagrams such as this are often mapped out experimentally by observing the cooling curve (temperature as a function of time) along isopleths of various compositions. this procedure is thermal analysis. a break in the slope of a cooling curve at a particular temperature indicates the system point has moved from a. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is.
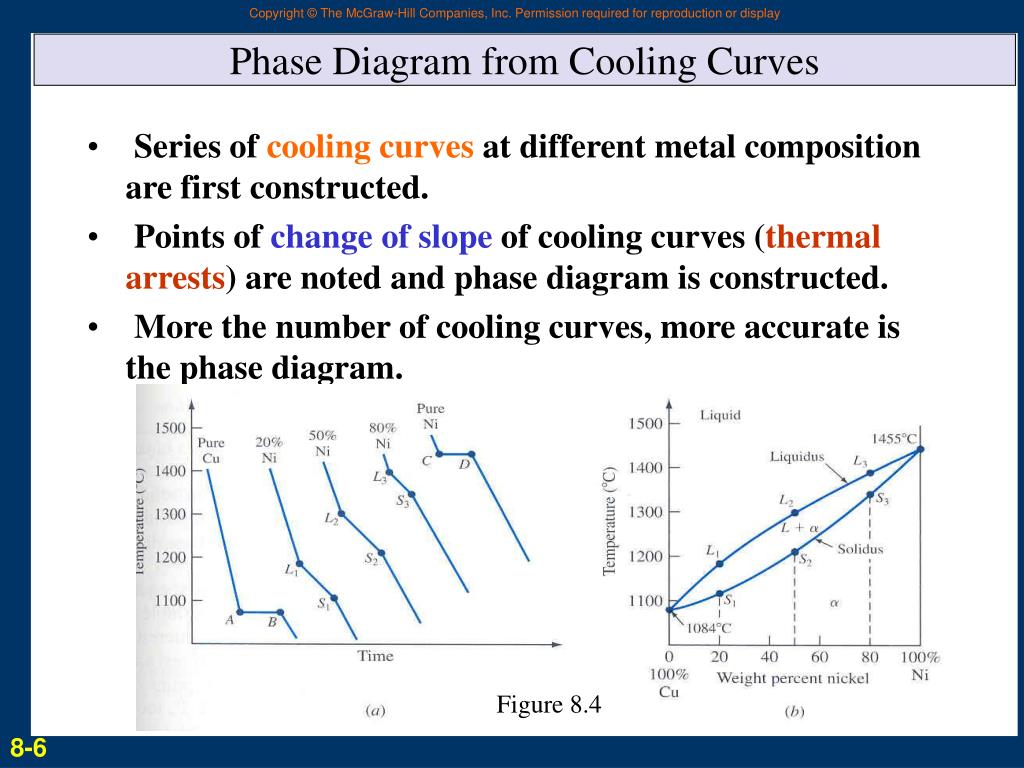
Cooling Curve Phase Diagram Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A typical phase diagram for a pure substance is shown in figure 11.5.1. figure 11.5.1. the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. to illustrate the utility of these plots, consider the phase diagram for water shown in figure 11.5.2.

Digging Into Phase Diagrams Cooling Curves Physical Chemistry We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A typical phase diagram for a pure substance is shown in figure 11.5.1. figure 11.5.1. the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. to illustrate the utility of these plots, consider the phase diagram for water shown in figure 11.5.2.

The Complete Guide To Understanding Cooling Curve Phase Diagrams

Intermolecular Forces Phase Diagrams Heating And Cooling Curves

Comments are closed.