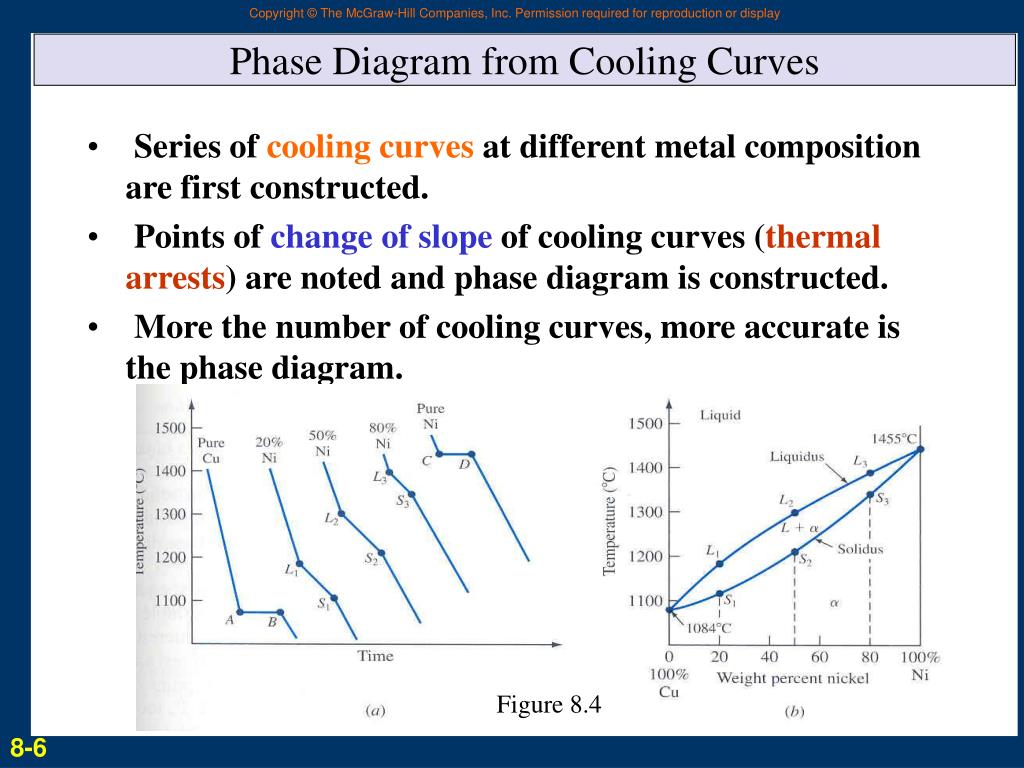
How To Convert Cooling Curves To Phase Diagrams

Digging Into Phase Diagrams Cooling Curves Physical Chemistry A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa.

Heating And Cooling Curves Overview Examples Expii A binary two phase system has two degrees of freedom. at a given t and p, each phase must have a fixed composition. we can calculate the liquid composition by rearranging eq. 13.2.4: xa = p − p ∗ b p ∗ a − p ∗ b the gas composition is then given by ya = pa p = xap ∗ a p = (p − p ∗ b p ∗ a − p ∗ b)p ∗ a p. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. On completion of this tlp you should: understand the thermodynamic principles behind free energy curves. understand how free energy curves relate to equilibrium phase diagrams. be able to construct a binary phase diagram from cooling curves. be able to use phase diagrams to predict the composition and volume fraction of phases. 12.1: introduction.
Cooling Curve Phase Diagram Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. On completion of this tlp you should: understand the thermodynamic principles behind free energy curves. understand how free energy curves relate to equilibrium phase diagrams. be able to construct a binary phase diagram from cooling curves. be able to use phase diagrams to predict the composition and volume fraction of phases. 12.1: introduction. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. Be able to construct a binary phase diagram from cooling curves; be able to use phase diagrams to predict the composition and volume fraction of phases; introduction. the phase diagram is a crucial part of metallurgy it shows the equilibrium states of a mixture, so that given a temperature and composition, it is possible to calculate which.

Cooling Curve Diagram At Georgia Correll Blog Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. Be able to construct a binary phase diagram from cooling curves; be able to use phase diagrams to predict the composition and volume fraction of phases; introduction. the phase diagram is a crucial part of metallurgy it shows the equilibrium states of a mixture, so that given a temperature and composition, it is possible to calculate which.

Comments are closed.