How To Determine Orders Of Reaction The Rate Equation And A Rate

How To Determine Orders Of Reaction The Rate Equation And A Rate Either the differential rate law (equation 14.19) or the integrated rate law (equation 14.21) can be used to determine whether a particular reaction is first order. graphs of a first order reaction. the expected shapes of the curves for plots of reactant concentration versus time (top) and the natural logarithm of reactant concentration versus. If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall.
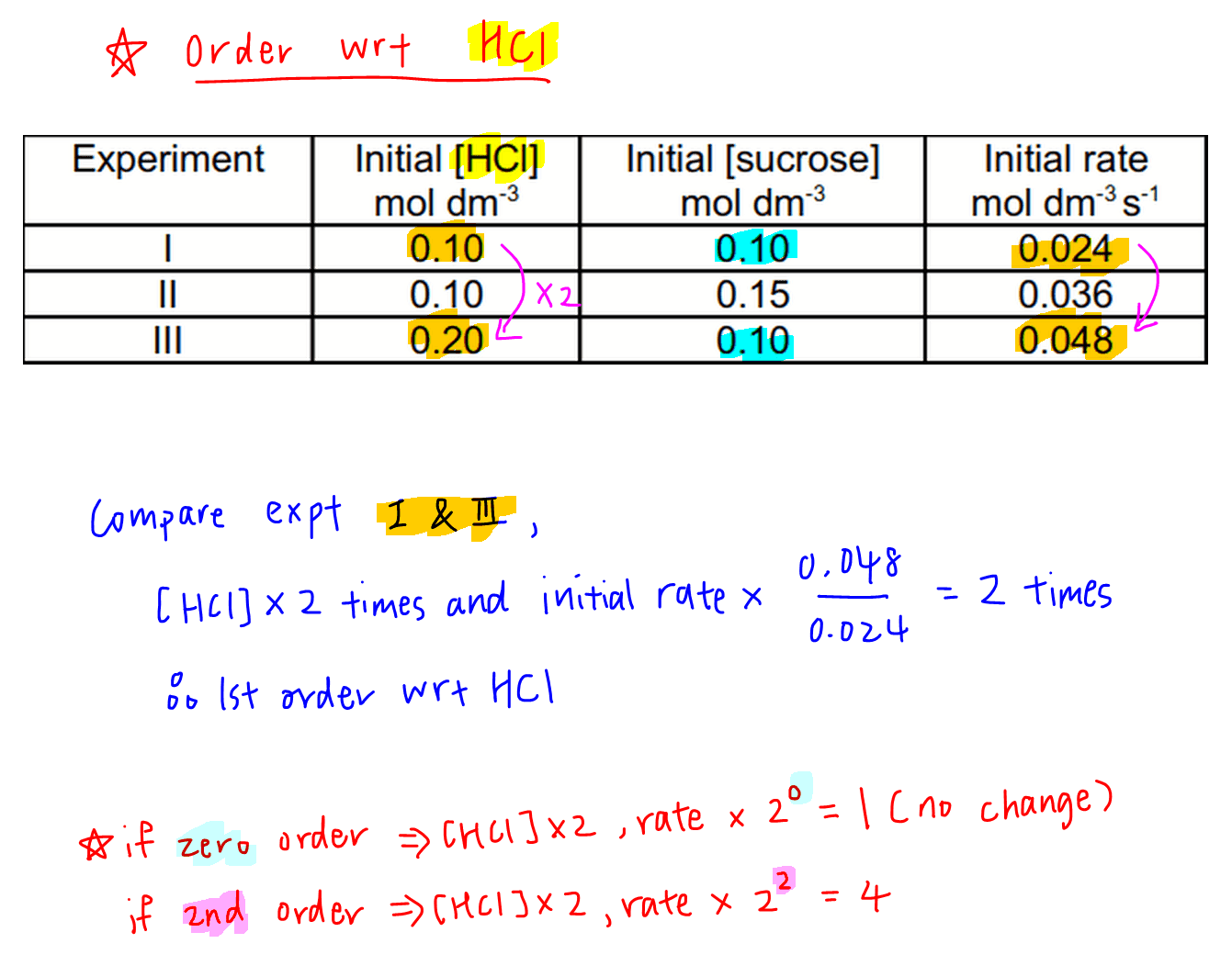
Determine That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction. A → products. rate = k[a]n. where k is the rate constant and n is the reaction order. our objective is to determine the reaction order by calculating the n from a set of experiments. keep in mind that: if n = 0, the reaction is zero order, and the rate is independent of the concentration of a. if n = 1, the reaction is first order, and the. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. for each unique chemical reaction, rate laws can be written at a rate law equation to show how the concentrations of reactants affect the rate of the reaction. Rate laws and reaction order. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. for example, the rate of the gas phase decomposition of dinitrogen pentoxide. 2n2o5 → 4no2 o2 (17.1.9) (17.1.9) 2 n 2 o 5 → 4 n o 2 o 2.
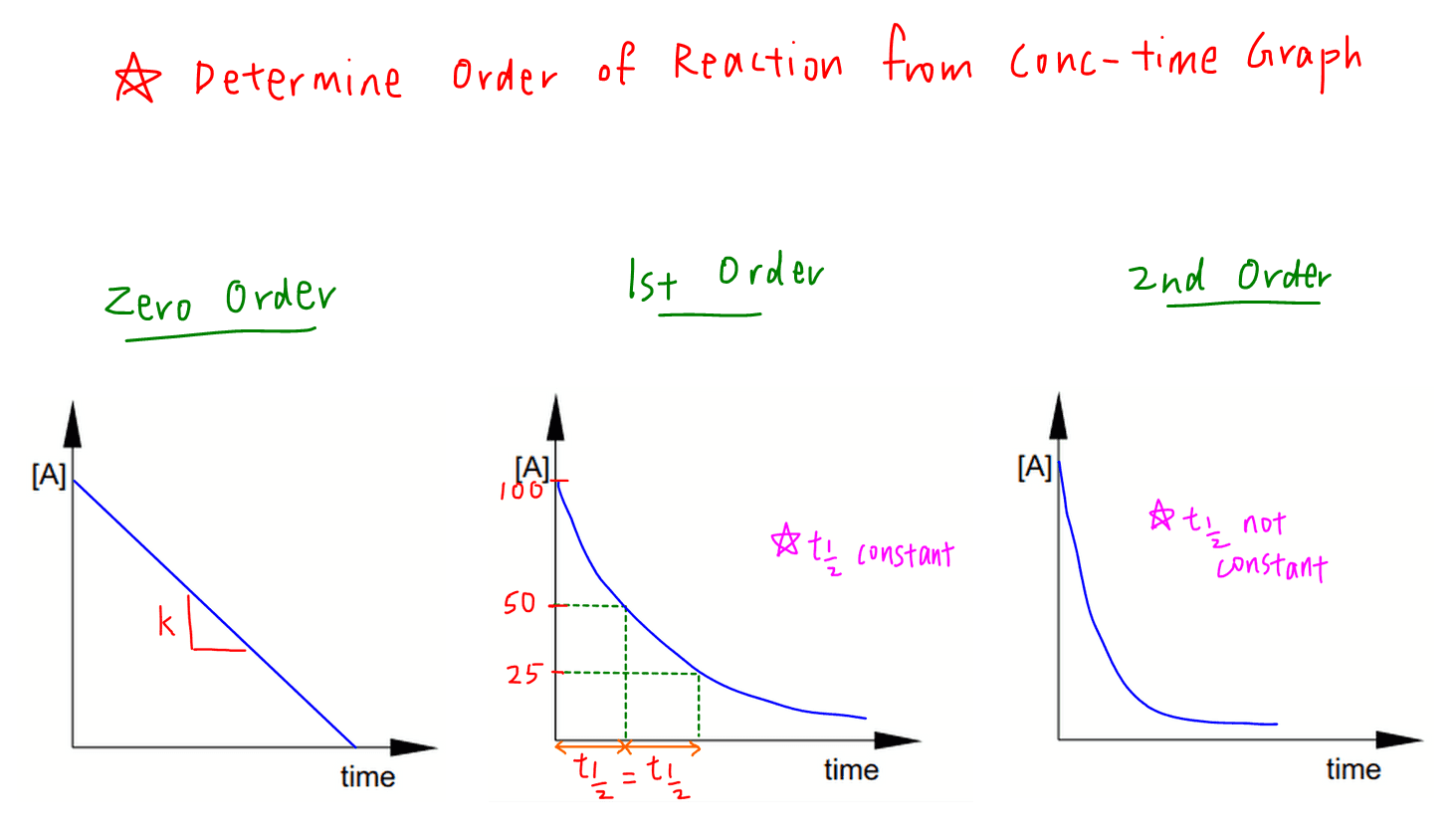
Rate Equation And Order Of Reaction The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. for each unique chemical reaction, rate laws can be written at a rate law equation to show how the concentrations of reactants affect the rate of the reaction. Rate laws and reaction order. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. for example, the rate of the gas phase decomposition of dinitrogen pentoxide. 2n2o5 → 4no2 o2 (17.1.9) (17.1.9) 2 n 2 o 5 → 4 n o 2 o 2. The rate law for this reaction is written as: rate = k[a]m[b]n rate = k [a] m [b] n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though. Example: write rate equation for reaction between a and b where a is 1st order and b is 2nd order. r = k[a][b]2 overall order is 3 calculate the unit of k unit of k = mol 2dm6s 1 1. rearrange rate equation to give k as subject k = rate [a][b]2 2. insert units and cancel k = mol dm 3s 1 mol dm 3.(moldm 3)2 3. simplify fraction k = s 1 mol2dm 6 n.
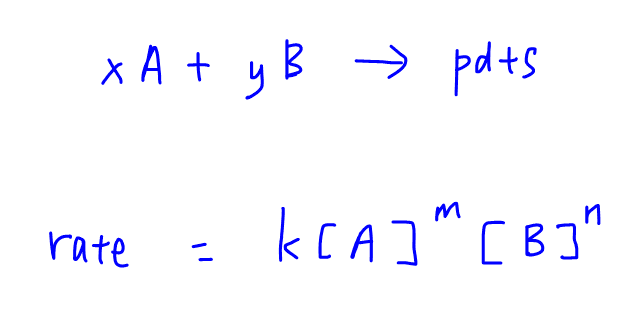
How To Calculate Rate Of Reaction In Chemistry The rate law for this reaction is written as: rate = k[a]m[b]n rate = k [a] m [b] n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though. Example: write rate equation for reaction between a and b where a is 1st order and b is 2nd order. r = k[a][b]2 overall order is 3 calculate the unit of k unit of k = mol 2dm6s 1 1. rearrange rate equation to give k as subject k = rate [a][b]2 2. insert units and cancel k = mol dm 3s 1 mol dm 3.(moldm 3)2 3. simplify fraction k = s 1 mol2dm 6 n.

Comments are closed.