How To Find The Rate Law And Rate Constant K

How To Determine The Units Of The Rate Constant K Chemical Kinetics The rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the exponents in a rate law describe the effects of the reactant concentrations on the reaction rate and define the reaction order. consider a reaction for which the rate law is: \[\ce{rate}=k[a]^m[b]^n \nonumber \]. Finding the rate law, rate constant and the rate constant units is all explained in a few simple steps. this question is a common exam question and in this v.

Determine The Rate Constant K For A Reaction Youtube The general rate law for the reaction is given in equation 14.3.12. we can obtain m or n directly by using a proportion of the rate laws for two experiments in which the concentration of one reactant is the same, such as experiments 1 and 3 in table 14.3.3. rate1 rate3 = k[a1]m[b1]n k[a3]m[b3]n. The rate law for this reaction is written as: rate = k[a]m[b]n rate = k [a] m [b] n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. for each unique chemical reaction, rate laws can be written at a rate law equation to show how the concentrations of reactants affect the rate of the reaction. For the reaction given by 2no o2 → 2no2, the rate equation is: rate = k [no]2[o2] find the overall order of the reaction and the units of the rate constant. the overall order of the reaction = sum of exponents of reactants in the rate equation = 2 1 = 3. the reaction is a third order reaction.

Chemistry Chemical Kinetics 15 Of 30 Finding Rate Law Rate The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. for each unique chemical reaction, rate laws can be written at a rate law equation to show how the concentrations of reactants affect the rate of the reaction. For the reaction given by 2no o2 → 2no2, the rate equation is: rate = k [no]2[o2] find the overall order of the reaction and the units of the rate constant. the overall order of the reaction = sum of exponents of reactants in the rate equation = 2 1 = 3. the reaction is a third order reaction. The rate law for this reaction is written as: rate = k[a]m[b]n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though they can be fractions. One way is to use the method of initial rates. a rate law shows how a change in concentration affects the rate. the equation for a component a is. rate = k[a]m, where m is the order of the reaction. zero order. rate = k[a]0 = k. the rate does not depend on the concentration. whatever you do to the concentration, the rate will not change.
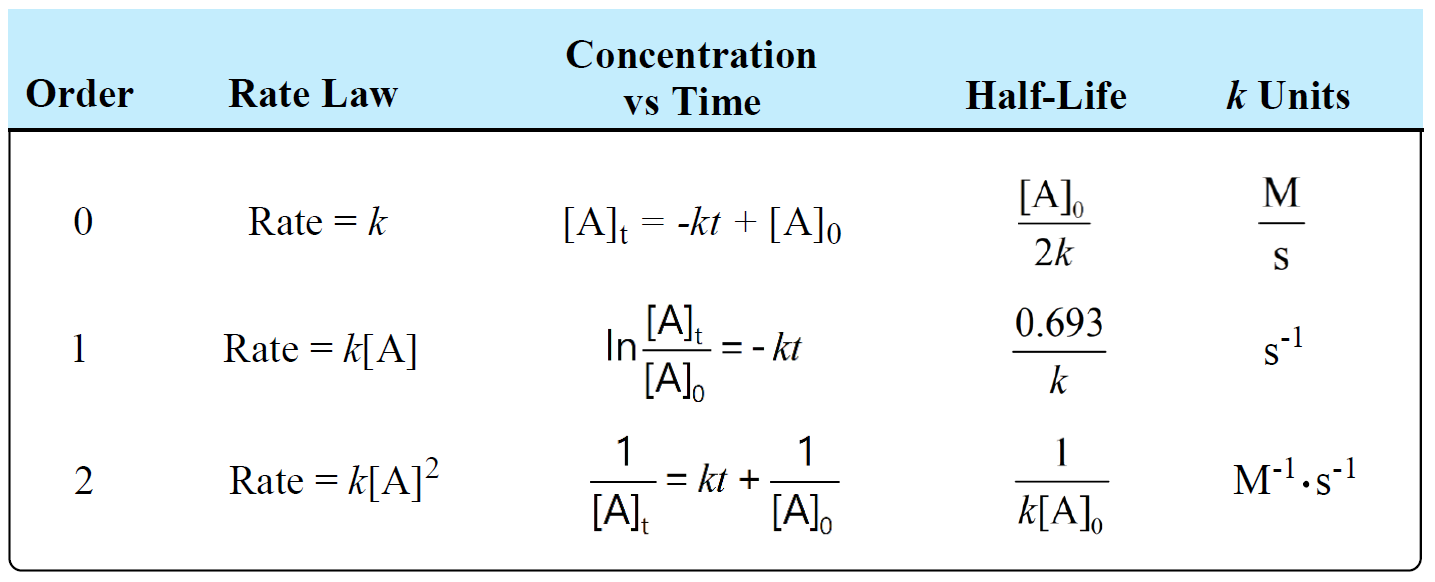
Units Of Rate Constant K Chemistry Steps The rate law for this reaction is written as: rate = k[a]m[b]n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though they can be fractions. One way is to use the method of initial rates. a rate law shows how a change in concentration affects the rate. the equation for a component a is. rate = k[a]m, where m is the order of the reaction. zero order. rate = k[a]0 = k. the rate does not depend on the concentration. whatever you do to the concentration, the rate will not change.
.PNG)
Rate Laws Presentation Chemistry

How To Calculate Rate Constant K Of Reaction Rates Youtube

Comments are closed.