Integrated Rate Laws Zero First Second Order Reactions Chemical

Integrated Rate Laws Zero First Second Order Reactions Chemical Second order reaction: rate = − Δ[a] Δt = k[a]2 1 [a] = 1 [a]0 kt. the reaction rate of a zeroth order reaction is independent of the concentration of the reactants. the reaction rate of a first order reaction is directly proportional to the concentration of one …. For zero order reactions, the differential rate law is: rate = k[a]0 = k. a zero order reaction thus exhibits a constant reaction rate, regardless of the concentration of its reactants. the integrated rate law for a zero order reaction also has the form of the equation of a straight line: [a] = − kt [a]0 y = mx b.
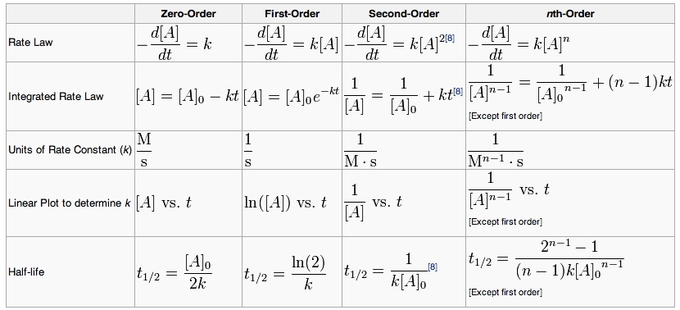
The Integrated Rate Law Introduction To Chemistry Integrated rate laws. most orders of reaction are zero, first or second. table \(\pageindex{1}\) gives the solutions to the integrated rate laws, and you need to know these solutions for zero, first and second order reactions. note, there is a form of each order of reaction that follows the equation of a straight line (y=mx b). For purposes of discussion, we will focus on the resulting integrated rate laws for first , second , and zero order reactions. first order reactions. integration of the rate law for a simple first order reaction (rate = [latex]k[a][ latex]) results in an equation describing how the reactant concentration varies with time:. Equations for both differential and integrated rate laws and the corresponding half lives for zero , first , and second order reactions are summarized in table 12.2. summary of rate laws for zero , first , and second order reactions. For purposes of discussion, we will focus on the resulting integrated rate laws for first , second , and zero order reactions. first order reactions an equation relating the rate constant k to the initial concentration [ a ] 0 and the concentration [ a ] t present after any given time t can be derived for a first order reaction and shown to be:.

Ppt Summary Of The Kinetics Of Zero Order First Order And Second Equations for both differential and integrated rate laws and the corresponding half lives for zero , first , and second order reactions are summarized in table 12.2. summary of rate laws for zero , first , and second order reactions. For purposes of discussion, we will focus on the resulting integrated rate laws for first , second , and zero order reactions. first order reactions an equation relating the rate constant k to the initial concentration [ a ] 0 and the concentration [ a ] t present after any given time t can be derived for a first order reaction and shown to be:. Perform integrated rate law calculations for zero , first , and second order reactions; define half life and carry out related calculations; identify the order of a reaction from concentration time data; the rate laws we have seen thus far relate the rate and the concentrations of reactants. we can also determine a second form of each rate law. The graph on the left shows [a] vs t plots for a zero order (red line), first order (gold line), and second order (blue line) reaction. the graph in the middle shows ln [a] vs t plots for each reaction order, and the graph on the right shows 1 [a] vs t plots for each reaction order. note: in the first order plot, the y axis is labeled as "ln [a]" .

Integrated Rate Law Graphs Chemistry Community Perform integrated rate law calculations for zero , first , and second order reactions; define half life and carry out related calculations; identify the order of a reaction from concentration time data; the rate laws we have seen thus far relate the rate and the concentrations of reactants. we can also determine a second form of each rate law. The graph on the left shows [a] vs t plots for a zero order (red line), first order (gold line), and second order (blue line) reaction. the graph in the middle shows ln [a] vs t plots for each reaction order, and the graph on the right shows 1 [a] vs t plots for each reaction order. note: in the first order plot, the y axis is labeled as "ln [a]" .

Comments are closed.