International Regulatory Cooperation To Improve Global Health Ema
International Regulatory Cooperation To Improve Global Health Ema The agency published 15 news announcements to inform the public about joint icmra initiatives and activities, such as vaccine confidence, transparency and data integrity and regulatory flexibility. as a result, icmra was mentioned in 1.4m media articles published globally from january to december 2021. six new members joined icmra in 2021. These assessments are carried out in cooperation with the world health organization (who) and aim to increase access by patients in low and middle income countries (lmics) to high quality, safe and effective medicines. the overall goal of this procedure is to contribute to improving global health. 'effective medicine regulation plays a crucial.

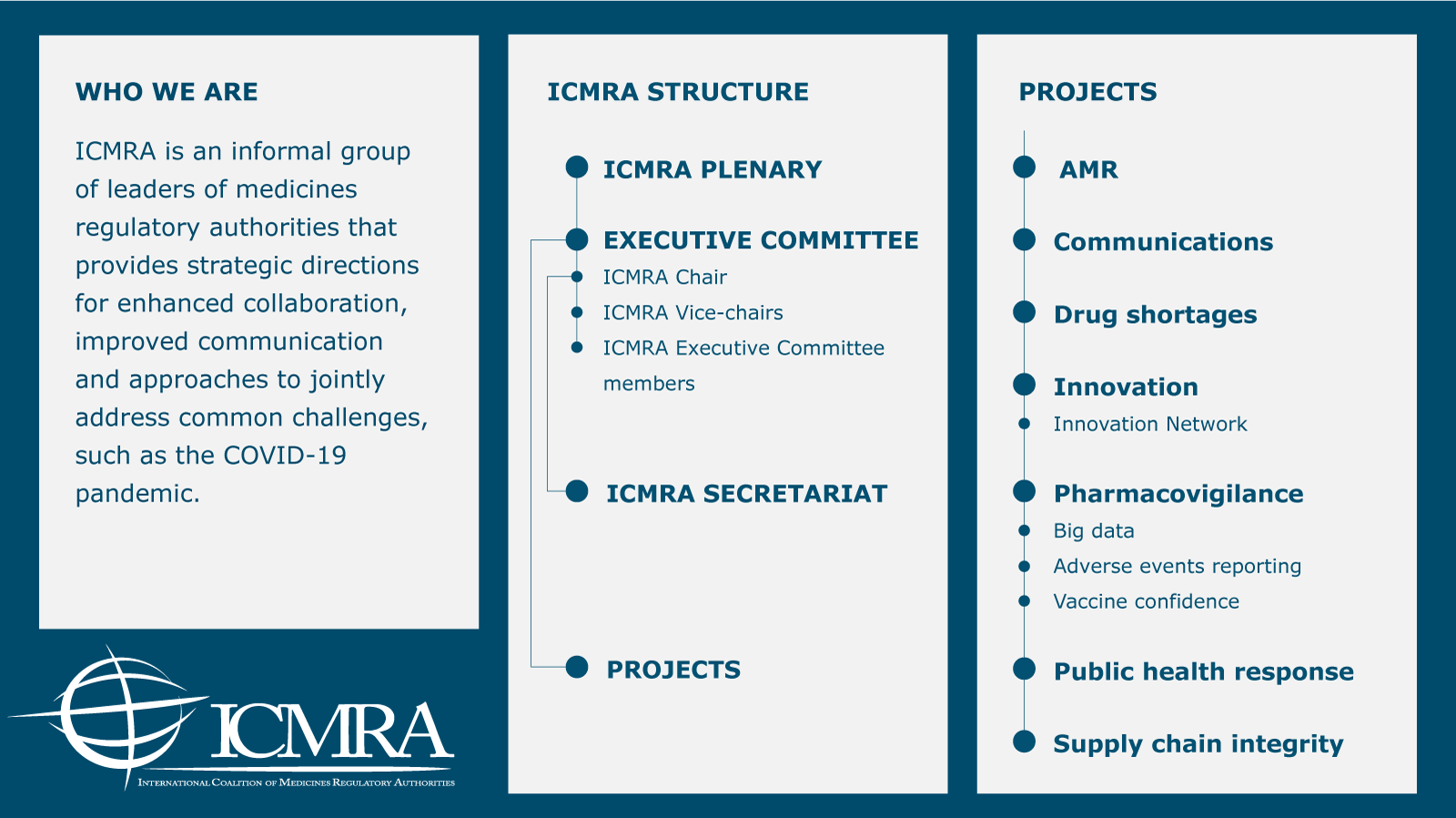
International Regulatory Cooperation To Improve Global Health Ema Icmra acts as a forum to support international cooperation among medicines regulatory authorities. the coalition aims to: identify ways to better use existing initiatives and resources; develop strategies to address current and emerging challenges in global human medicine regulation, such as the growing complexity of globalised supply chains;. Ema experts meet with african regulators to discuss opportunities for collaboration. how to improve the availability of high quality, safe and effective medicines to patients in countries beyond europe and how to make better use of existing tools? these were two of the questions discussed at a workshop jointly organised by the european medicines agency […]. The considerable heterogeneity of the global regulatory landscape and lack of convergence and harmonization in regulatory requirements with respect to evidence, review, and authorization processes.”15 as part of the global effort to quickly develop and distribute covid 19 vaccines, the ema launched a pilot. I. n january 2019, the european medicines agency (ema) published the regulatory science to 2025: strategic reflection paper for public consultation. it comprises sections on both human and veterinary medicines and discusses the importance of international regulatory science cooperation. in this reflection paper, ema stresses the need to foster.
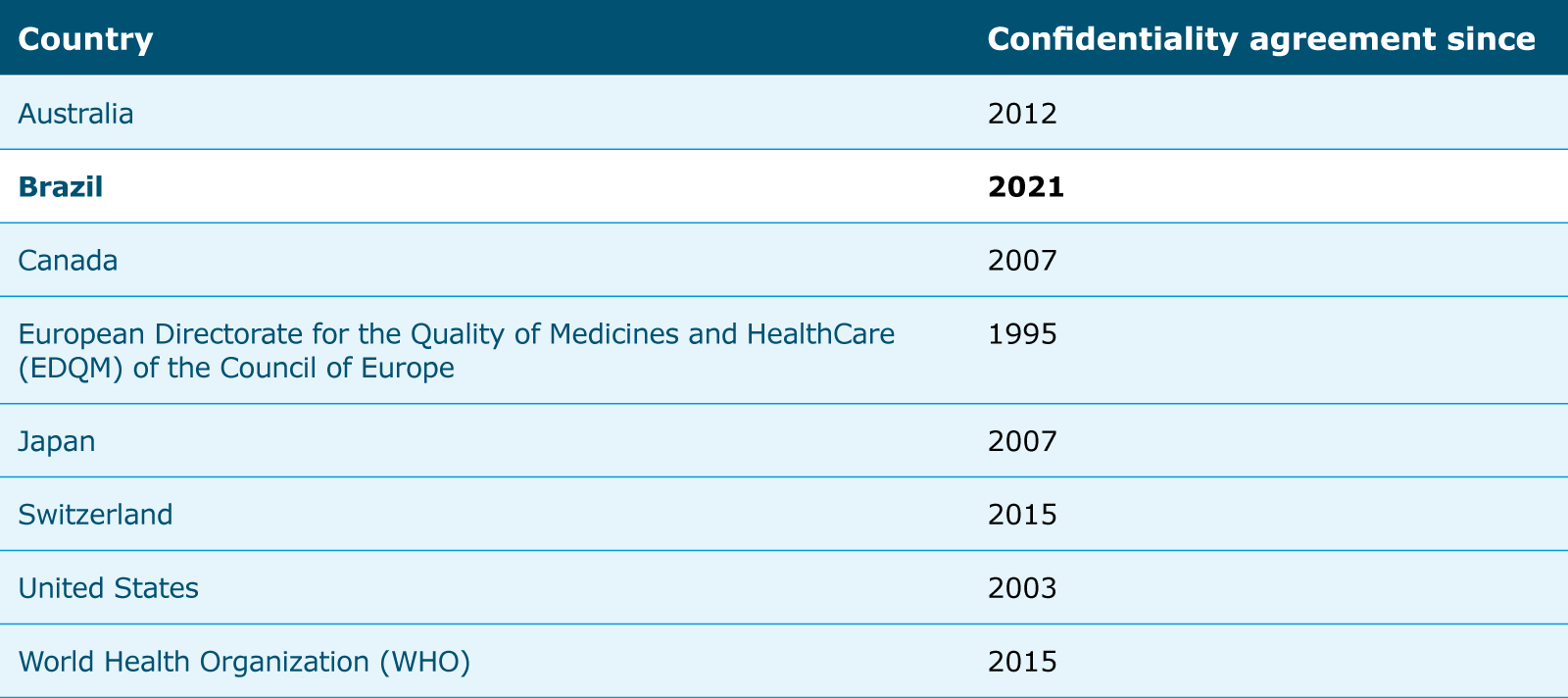
International Regulatory Cooperation To Improve Global Health Ema The considerable heterogeneity of the global regulatory landscape and lack of convergence and harmonization in regulatory requirements with respect to evidence, review, and authorization processes.”15 as part of the global effort to quickly develop and distribute covid 19 vaccines, the ema launched a pilot. I. n january 2019, the european medicines agency (ema) published the regulatory science to 2025: strategic reflection paper for public consultation. it comprises sections on both human and veterinary medicines and discusses the importance of international regulatory science cooperation. in this reflection paper, ema stresses the need to foster. The icmra was founded as an international, non political organisation in 2013 at the initiative of the heads of the regulatory authorities, the heads of agencies (hoa). it currently comprises 24 members, 15 associated authorities and the who as an observer. swissmedic is a member of the icmra and supports the initiative in working groups such. “medicines regulatory harmonization: international collaboration as a key to improve public health.” medicine access @ point of care. 1 jan. 2017. web. international alliance of patients’ organizations. “sovereignty preventing global drug regulation harmonization international conference of drug regulatory authorities.”.
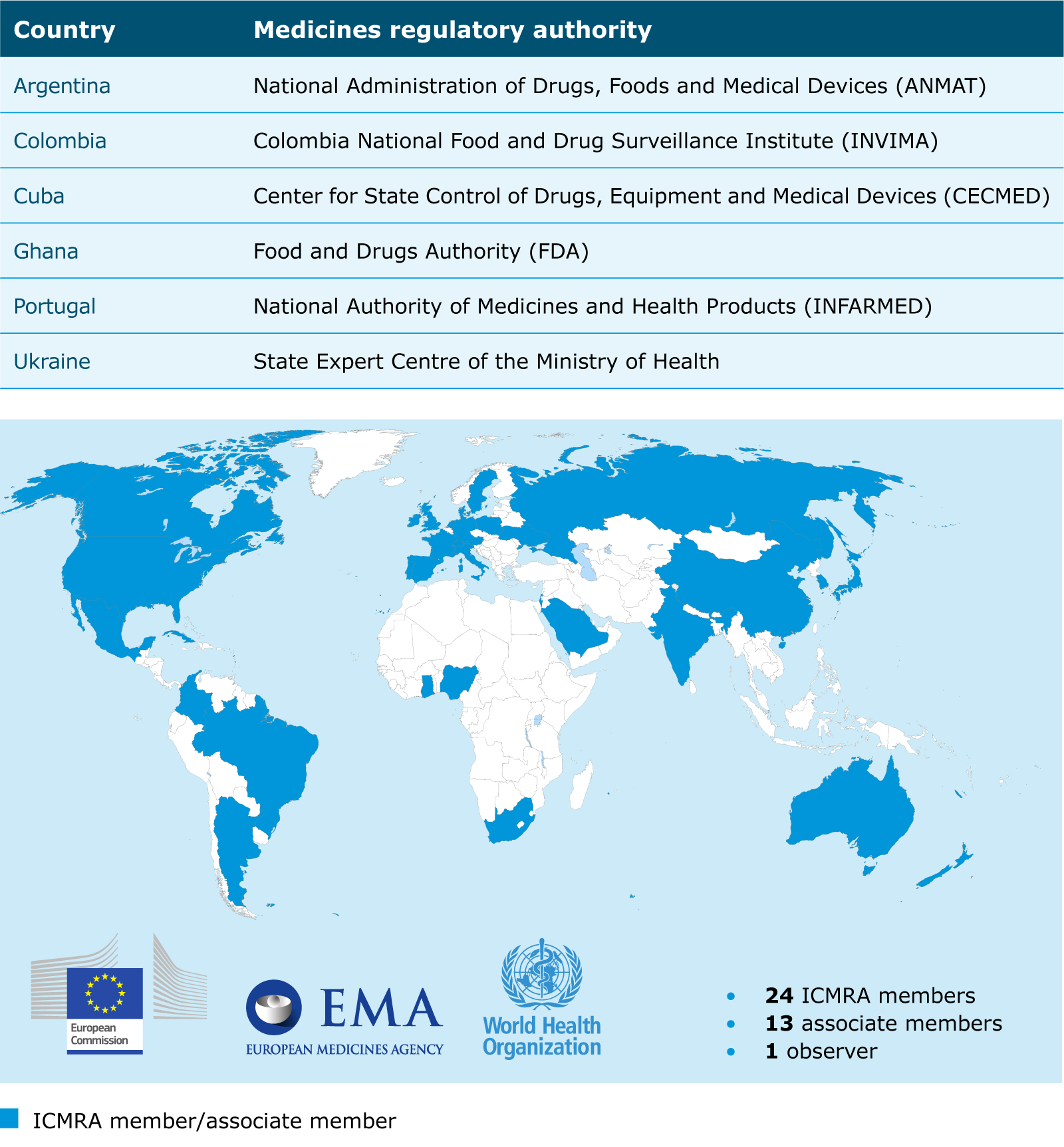
International Regulatory Cooperation To Improve Global Health Ema The icmra was founded as an international, non political organisation in 2013 at the initiative of the heads of the regulatory authorities, the heads of agencies (hoa). it currently comprises 24 members, 15 associated authorities and the who as an observer. swissmedic is a member of the icmra and supports the initiative in working groups such. “medicines regulatory harmonization: international collaboration as a key to improve public health.” medicine access @ point of care. 1 jan. 2017. web. international alliance of patients’ organizations. “sovereignty preventing global drug regulation harmonization international conference of drug regulatory authorities.”.

Homepage Ema Annual Report 2021

Comments are closed.