Lewis Diagrams Made Easy How To Draw Lewis Dot Structures
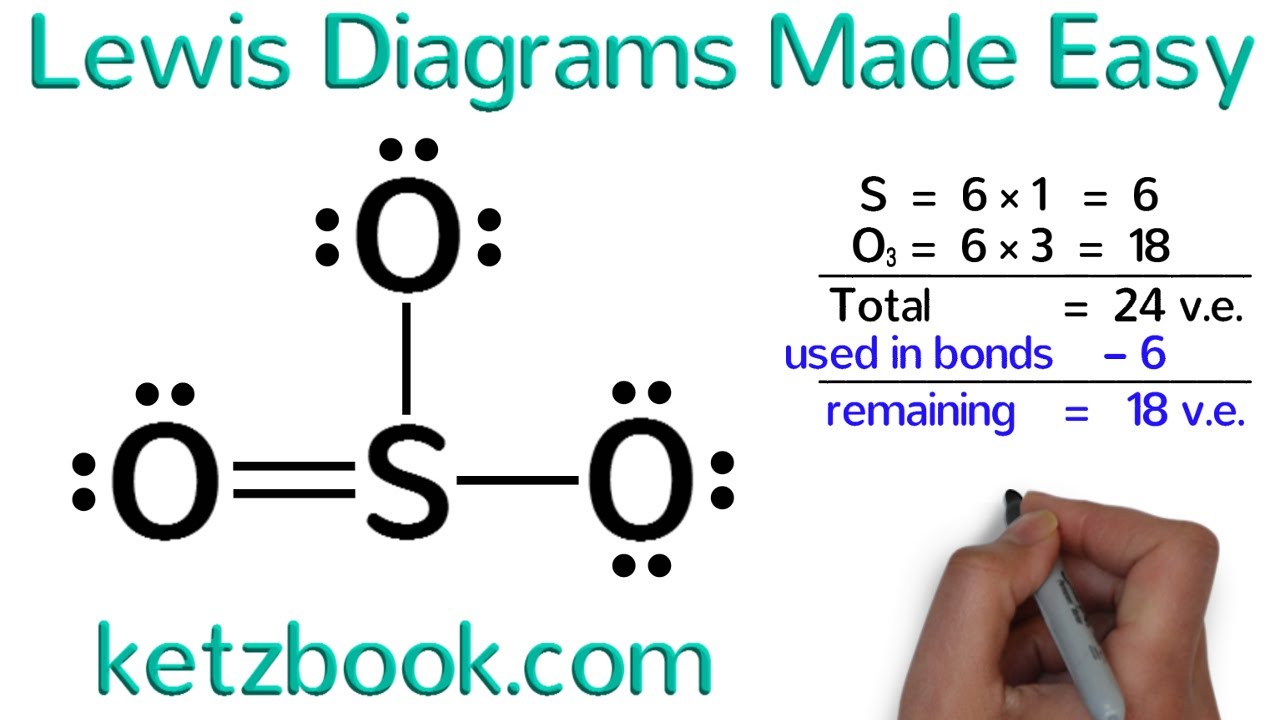
Lewis Diagrams Made Easy How To Draw Lewis Dot Structures Youtube This is a whiteboard animation tutorial on how to draw lewis structures of molecules. this is my second tutorial in the series. the first video includes le. A video tutorial for how to draw lewis structures in five steps. more lewis structures help at breslyn.org (and get all my free chem video guid.

How To Draw A Lewis Structure Torrey glenn, city college of san francisco. 9.3: drawing lewis structures is shared under a license and was authored, remixed, and or curated by libretexts. a lewis structure is a way to show how atoms share electrons when they form a molecule. lewis structures show all of the valence electrons in an atom or molecule. Chad explains and demonstrates exactly how to draw lewis dot structures in this lesson including numerous examples. the octet rule is introduced and explain. Examples for drawing lewis structures for covalent bonds . here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. reference the “how to draw a lewis dot structure” for a step by step guide. see the following lewis dot structure diagrams for a few covalent compounds. example 1. A lewis structure includes lines for covalent chemical bonds and dots for valence electrons or lone electron pairs. different ways to draw lewis structures. there is more than one “right” way to draw a lewis structure. if you are drawing the structures for a chemistry class, be sure to know what your instructor expects.

How To Draw A Lewis Structure Steps Examples for drawing lewis structures for covalent bonds . here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. reference the “how to draw a lewis dot structure” for a step by step guide. see the following lewis dot structure diagrams for a few covalent compounds. example 1. A lewis structure includes lines for covalent chemical bonds and dots for valence electrons or lone electron pairs. different ways to draw lewis structures. there is more than one “right” way to draw a lewis structure. if you are drawing the structures for a chemistry class, be sure to know what your instructor expects. Lewis structures (also known as lewis dot diagrams, electron dot diagrams,"lewis dot formula" lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. a lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. the lewis structure. Step 3: determine the number of bonds in the molecule. covalent bonds are formed when one electron from each atom forms an electron pair. step 2 tells how many electrons are needed and step 1 is how many electrons you have. subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to complete the octets.

A Simple Guide For Learning How To Draw Lewis Dot Structures Lewis structures (also known as lewis dot diagrams, electron dot diagrams,"lewis dot formula" lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. a lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. the lewis structure. Step 3: determine the number of bonds in the molecule. covalent bonds are formed when one electron from each atom forms an electron pair. step 2 tells how many electrons are needed and step 1 is how many electrons you have. subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to complete the octets.

Comments are closed.