National 5 Physics Properties Of Matter Heating Cooling Curves Theory

National 5 Physics Properties Of Matter Heating Cooling Curves A brief overview of heating and cooling curves from the properties of matter topic in the national 5 physics course. in particular, we explain the parts of. Particles increases with increasing temperature. the hotter the gas, the faster the gas particles. o require to take up more space. instructionsplace the boiling tubes of water and stearic acid in a beaker of heated water or water. th until the stearic acid is completely melted.place thermometers or temperature probes in the two boiling tubes.
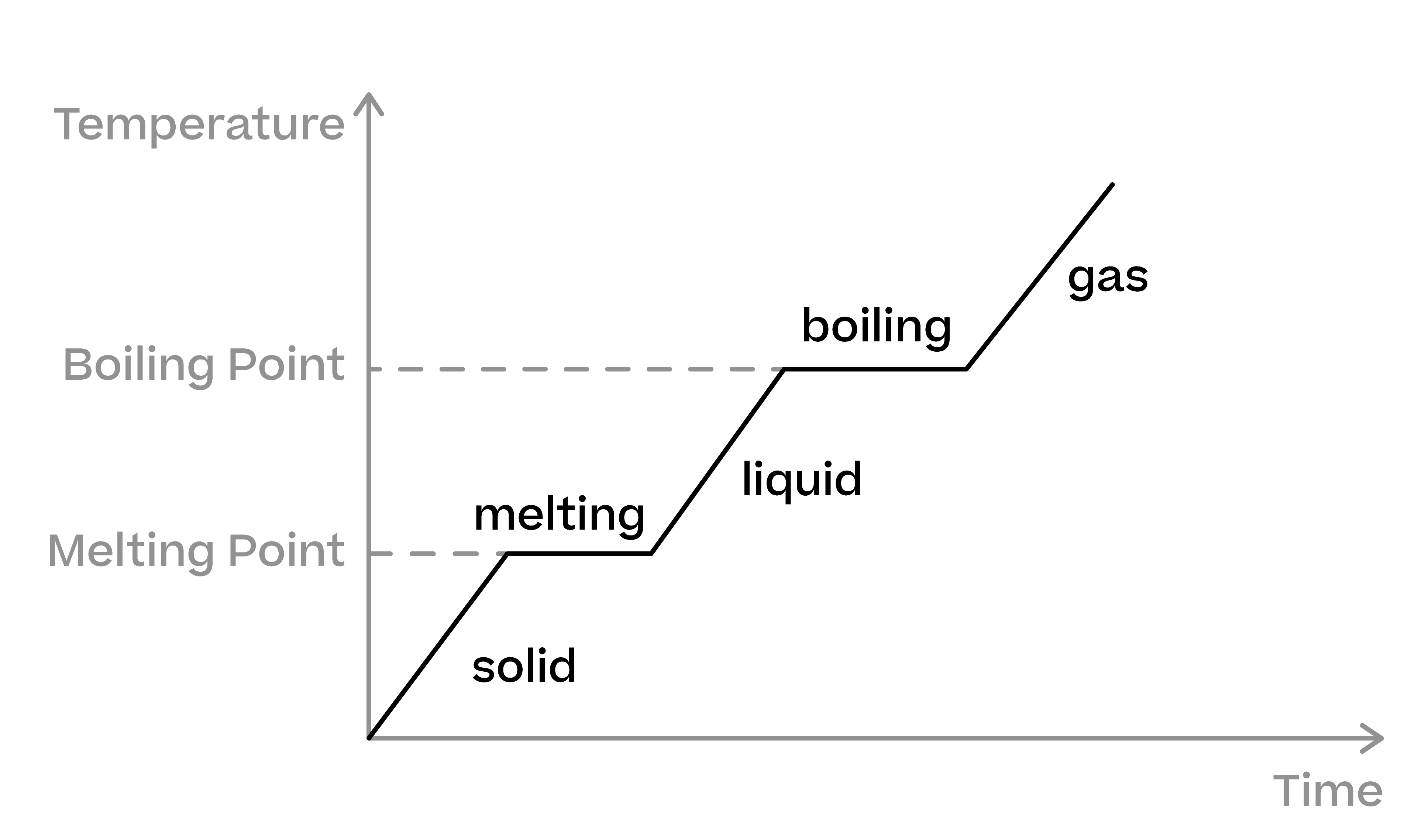
Heating And Cooling Curves Science Explanation Exercises Evulpo The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. Heating and cooling curves are graphical representations that show how the temperature of a substance changes as heat is added or removed over time. they illustrate the behavior of substances as they go through different states—solid, liquid, and gas. heating curve: this curve shows how the temperature of a substance increases as it absorbs heat. A brief overview of the difference between heat and temperature from the properties of matter topic in the national 5 physics course.thanks for watching! su. The properties of matter booklet in both word and pdf form. properties of matter 2022 pdf download. properties of matter 2022 word download. s3 n5 pressure. air pressure and pv h s3n5. here are a set of summary notes, i made a few changes and put them into a table rather than boxes to help the flow, not that anyone would know.

Heating And Cooling Curve Introduction Plus Kinetic And Potential A brief overview of the difference between heat and temperature from the properties of matter topic in the national 5 physics course.thanks for watching! su. The properties of matter booklet in both word and pdf form. properties of matter 2022 pdf download. properties of matter 2022 word download. s3 n5 pressure. air pressure and pv h s3n5. here are a set of summary notes, i made a few changes and put them into a table rather than boxes to help the flow, not that anyone would know. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Heating And Cooling Curve Chart For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Comments are closed.