Nh3 Balancing Chemical Reactions Ammonia Haber Bosch Process

Nh3 Balancing Chemical Reactions Ammonia Haber Bosch Process In this video we look at keeping chemical equations honest with the same number of atoms on each side of the equation.for more on chemical equations and chem. The haber process, [1] also called the haber–bosch process, is the main industrial procedure for the production of ammonia. [2][3] it converts atmospheric nitrogen (n 2) to ammonia (nh 3) by a reaction with hydrogen (h 2) using a finely divided iron metal catalyst: this reaction is slightly favorable in terms of enthalpy, but is disfavored in.
Solved Consider The Haber Bosch Process For The Synthesis Of Ammonia The traditional haber bosch process uses fossil fuel as a source to produce the required hydrogen. in recent times, there has been more focus on producing ammonia through many greener routes. the hydrogen required for the haber bosch reaction process is generated through the electrolysis of water. Truro school in cornwall. the haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. the process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. the reaction is reversible and the. In the haber bosch process, the exothermic reaction of hydrogen and nitrogen is favored by low temperatures and high pressures. however, on the fe based catalysts used in the industry, higher temperatures are required to dissociate n 2, which is the rate limiting step of the ammonia synthesis reaction. Carl bosch scaled the process and, in 1913, the first commercial haber–bosch plant opened 7. since, the haber–bosch process has been modified to make ammonia synthesis cleaner.
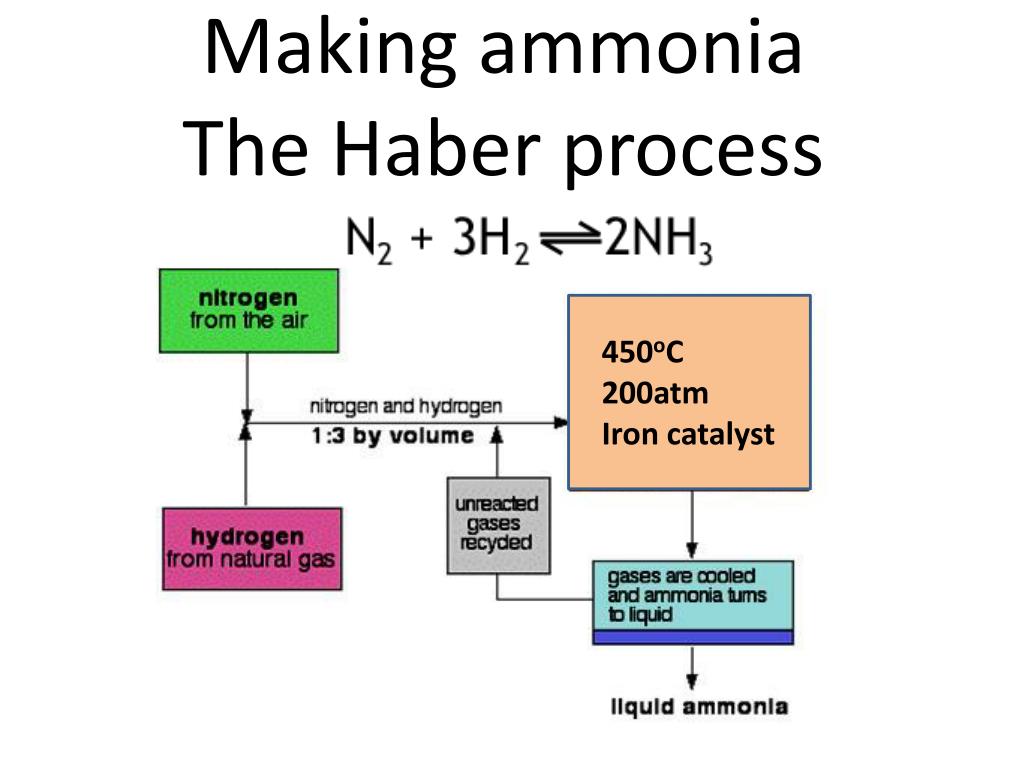
Ppt Making Ammonia The Haber Process Powerpoint Presentation Free In the haber bosch process, the exothermic reaction of hydrogen and nitrogen is favored by low temperatures and high pressures. however, on the fe based catalysts used in the industry, higher temperatures are required to dissociate n 2, which is the rate limiting step of the ammonia synthesis reaction. Carl bosch scaled the process and, in 1913, the first commercial haber–bosch plant opened 7. since, the haber–bosch process has been modified to make ammonia synthesis cleaner. Most modern ammonia synthesis catalysts used in the haber–bosch process are reported to achieve a conversion rate of around 10–15% operating in the range of 425–450 °c at pressures above 100 atm. from this it can be seen that if catalysts can be developed to increase the rate of reaction at temperatures lower than this then the. The haber bosch process converts atmospheric nitrogen (n 2) to ammonia (nh 3) by combining it with hydrogen (h 2). the process combines a single nitrogen molecule with 3 hydrogen molecules to produce 2 molecules of ammonia. the chemical equation for the haber bosch process is. n 2 3h 2 ⇌ 2nh 3. the ⇌ arrow in the above equation implies.
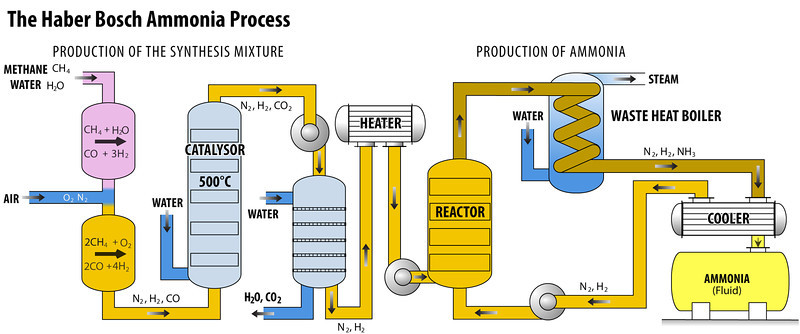
Haber Process Ammonia Manufacturing The Engineering Concepts Most modern ammonia synthesis catalysts used in the haber–bosch process are reported to achieve a conversion rate of around 10–15% operating in the range of 425–450 °c at pressures above 100 atm. from this it can be seen that if catalysts can be developed to increase the rate of reaction at temperatures lower than this then the. The haber bosch process converts atmospheric nitrogen (n 2) to ammonia (nh 3) by combining it with hydrogen (h 2). the process combines a single nitrogen molecule with 3 hydrogen molecules to produce 2 molecules of ammonia. the chemical equation for the haber bosch process is. n 2 3h 2 ⇌ 2nh 3. the ⇌ arrow in the above equation implies.
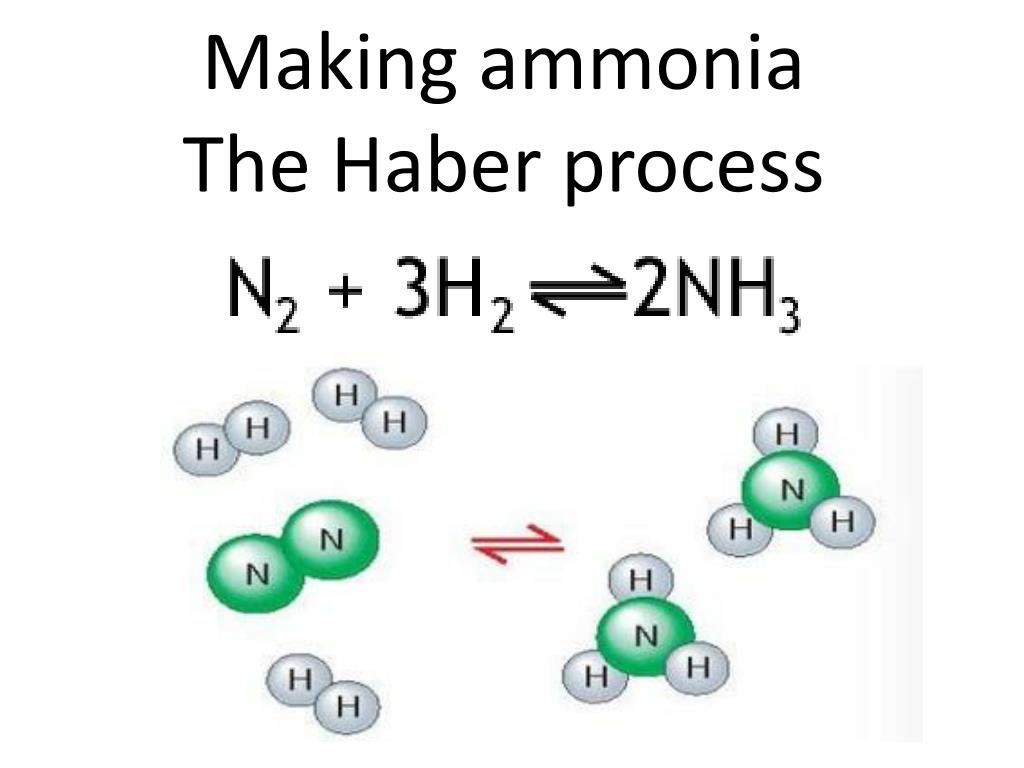
Ppt Making Ammonia The Haber Process Powerpoint Presentation Free

Haber Process Ammonia

Comments are closed.