Orders Of Reaction And Rate Equations
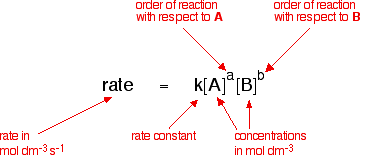
Orders Of Reaction And Rate Equations That means that that particular term disappears from the rate equation. the overall order of the reaction is found by adding up the individual orders. for example, if the reaction is first order with respect to both a and b (a = 1 and b = 1), the overall order is 2. we call this an overall second order reaction. Given the rate law equation: rate = k[a]1[b]2. 2. determine: a) the reaction order with respect to a, b) the reaction order with respect to b, and c) the total reaction order for the equation. 3. assuming the reaction occurs in one elementary step, propose a chemical equation using p as the symbol for your product.
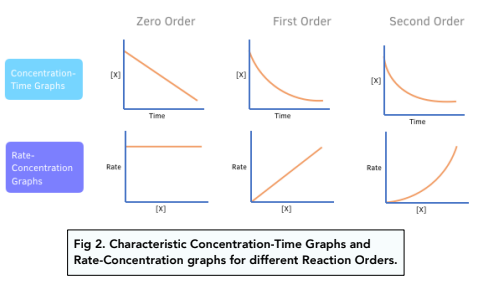
Rate Equations Determining Orders Of Reactions Graphically A Level Rate laws and reaction order. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. for example, the rate of the gas phase decomposition of dinitrogen pentoxide. 2n2o5 → 4no2 o2 (17.1.9) (17.1.9) 2 n 2 o 5 → 4 n o 2 o 2. If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall. The rate law for this reaction is written as: rate = k[a]m[b]n rate = k [a] m [b] n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though. Here, x = 1 and y = 2. the reaction is first order in a and second order in b. therefore, the overall reaction order is 1 2 = 3, or a third order reaction. while c is present in the reaction, its concentration does not appear in the rate law equation. the reaction is zero order in c, and the rate does not depend on its concentration.
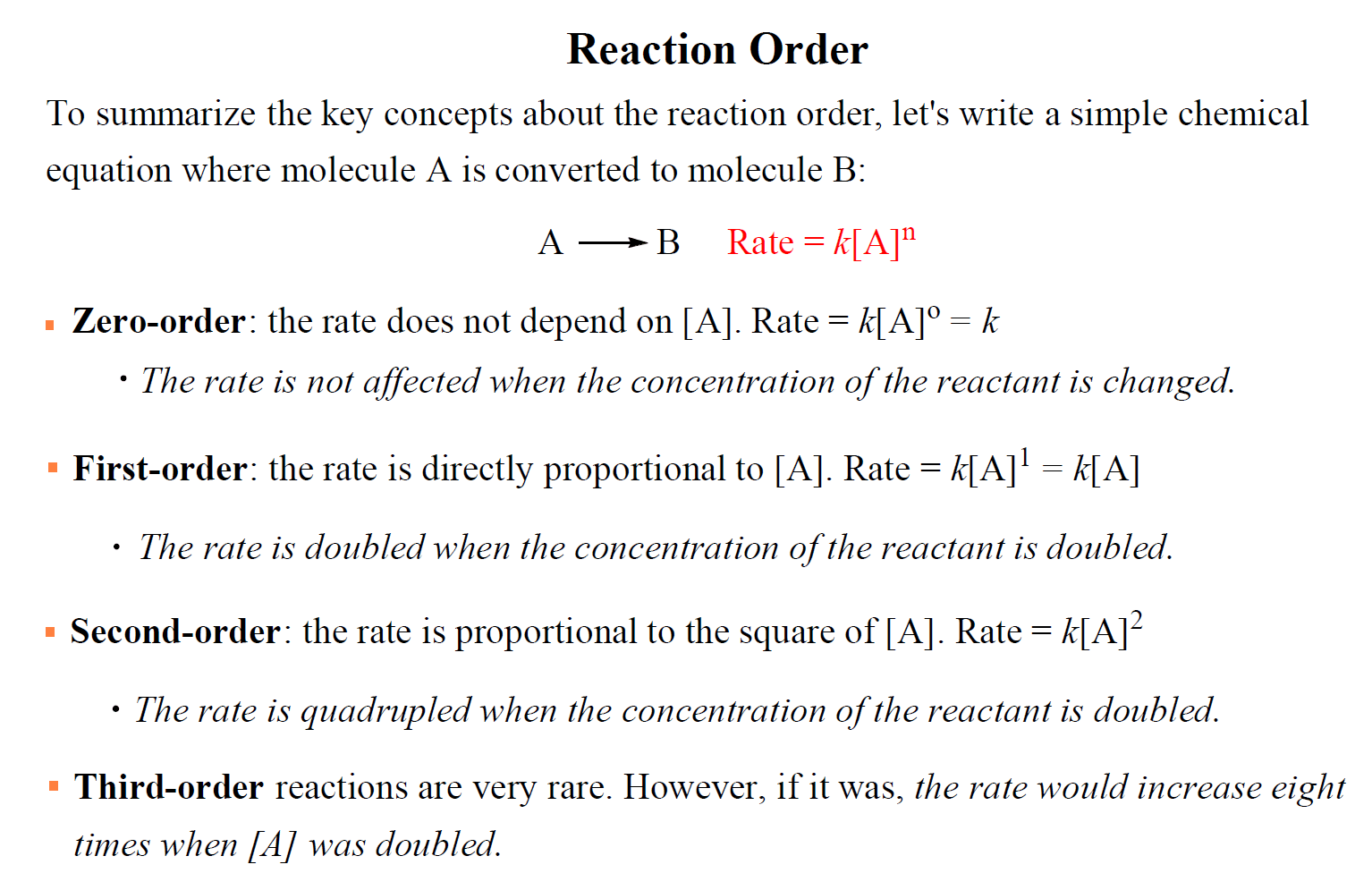
Rate Law And Reaction Order Chemistry Steps The rate law for this reaction is written as: rate = k[a]m[b]n rate = k [a] m [b] n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though. Here, x = 1 and y = 2. the reaction is first order in a and second order in b. therefore, the overall reaction order is 1 2 = 3, or a third order reaction. while c is present in the reaction, its concentration does not appear in the rate law equation. the reaction is zero order in c, and the rate does not depend on its concentration. Reaction is first order in a and zero order in b overall order = 1 0 = 1 usually written: rate = k[a] remember: the values of the reaction orders must be determined from experiment; they cannot be found by looking at the balanced reaction equation calculating units of k 1. rearrange rate equation to give k as subject k = rate [a] 2. The rate law for this reaction is written as: rate = k[a]m[b]n. in which [a] and [b] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a particular temperature. the exponents m and n are the reaction orders and are typically positive integers, though they can be fractions.

Comments are closed.