Pharmacovigilance Regulatory Health Authorities Agencies Around The

Pharmacovigilance Regulatory Health Authorities Agencies Around The Reference id: pharmatutor art 1316 introduction as the pharmaceutical industries throughout the world are moving ahead towards becoming more and more competitive, regulatory agencies are being established in various countries across the globe.regulatory authority and organizations are responsible in effective drug regulation required to ensure the safety, efficacy and quality of drugs, as well. Regulation and prequalification. progress towards enhancing regulatory practices related to medical products around the world is supported by capacity building, promoting regulatory convergence and harmonization. incidents and sf. laboratory networks and services. pharmacovigilance.

Regulatory Authorities In Pharmacovigilance Pv Drug Safety Academy Malaysia: national pharmaceutical regulatory agency. new zealand: ministry of health. new zealand: medicines and medical devices safety authority. new zealand: food safety authority. papua new guinea: department of health. philippines: department of health. philippines: national food authority. The european medicines agency (ema) coordinates the european union (eu) pharmacovigilance system and operates services and processes to support pharmacovigilance in the eu. updated on 6 june 2024: 'measuring the impact of pharmacovigilance activities' section. before a medicine is authorised for use, evidence of its safety and efficacy is. The international council for harmonization of technical requirements for pharmaceuticals for human use (ich) brings together the medicines regulatory authorities and pharmaceutical industry around the world. pharmacovigilance webinars and seminars. fda and mhra pharmacovigilance inspection readiness and management. this webinar provides. Due to the complexity of the number of regulatory authorities and their importance for the regulatory environment, we listed them alphabetically*: multi international organizations: world health organization. who regional office for europe. who regional office for africa. who regional office for the eastern mediterranean.
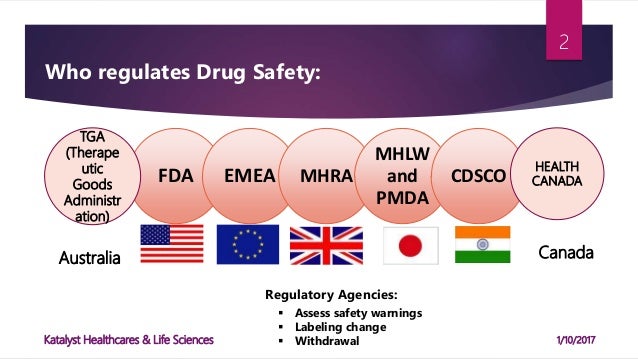
Pharmacovigilance Regulations Katalyst Hls The international council for harmonization of technical requirements for pharmaceuticals for human use (ich) brings together the medicines regulatory authorities and pharmaceutical industry around the world. pharmacovigilance webinars and seminars. fda and mhra pharmacovigilance inspection readiness and management. this webinar provides. Due to the complexity of the number of regulatory authorities and their importance for the regulatory environment, we listed them alphabetically*: multi international organizations: world health organization. who regional office for europe. who regional office for africa. who regional office for the eastern mediterranean. The european medicines agency (ema) is a member of the international coalition of medicines regulatory authorities (icmra). icmra is a voluntary, executive level entity of worldwide medicines regulatory authorities set up to provide strategic coordination, advocacy and leadership. Components of a good postmarketing report. description of adverse event. suspected and concomitant product therapy details (e.g., dose, dates of therapy) patient characteristics (e.g., age, sex.

Comments are closed.