Phase Changes Heats Of Fusion And Vaporization And Phase Diagrams
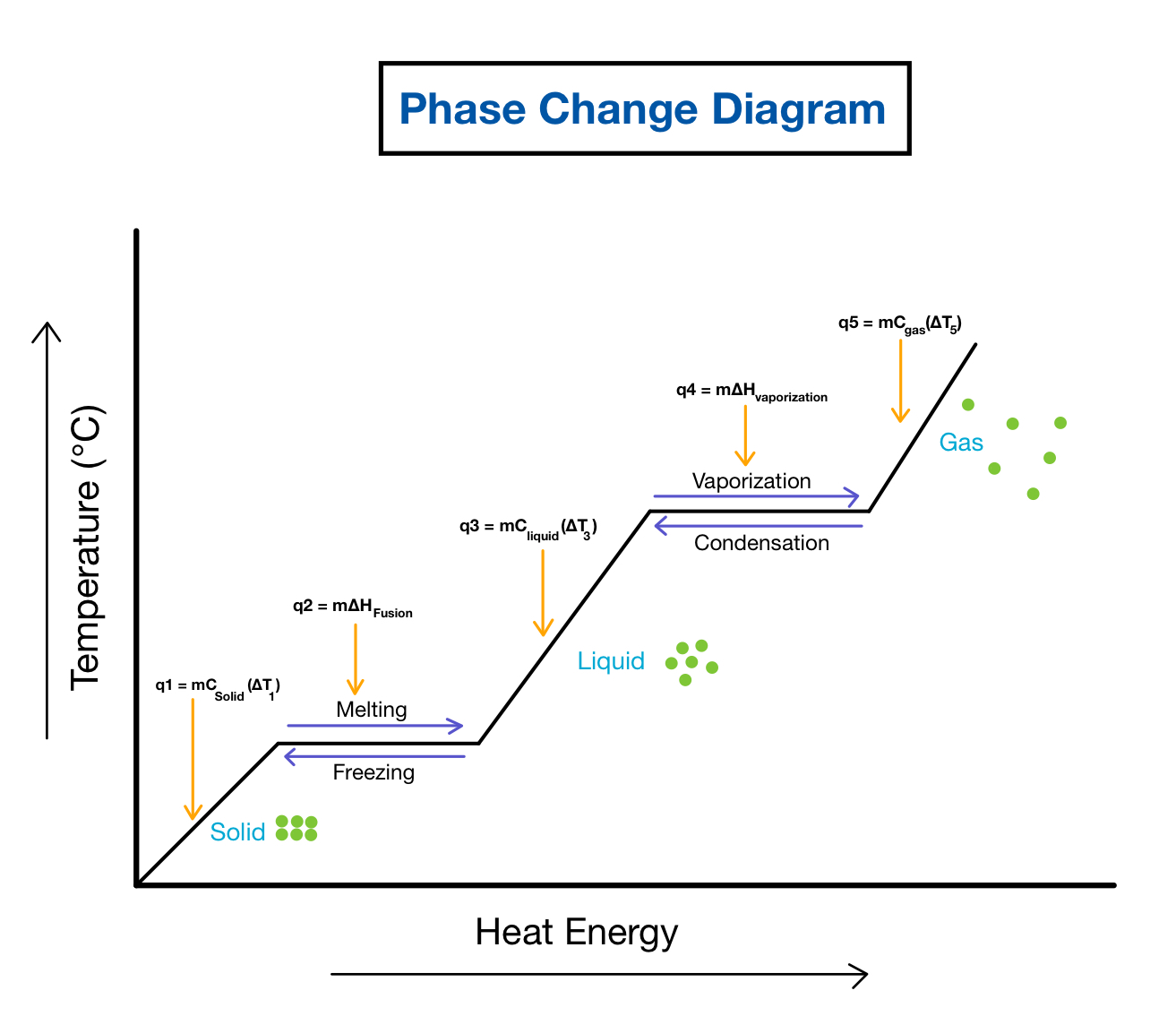
Phase Change Diagrams Overview Examples Expii What the heck is dry ice and why is it so spooky? learn this and more when we investigate phase changes and phase diagrams!watch the whole general chemistry. Phase changes, heats of fusion and vaporization, and phase diagrams. professor dave explains. 478.
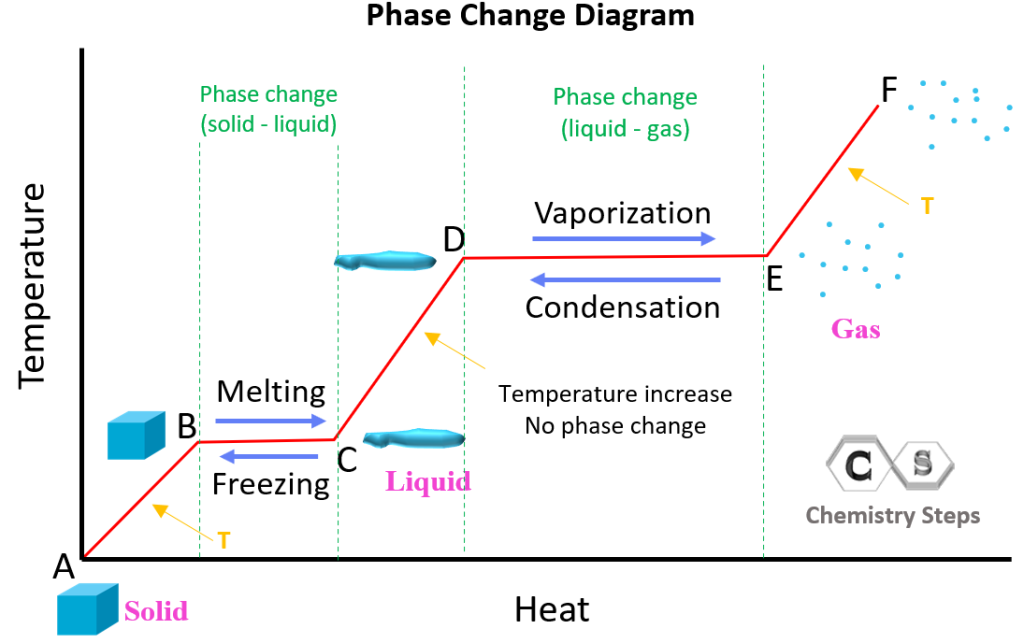
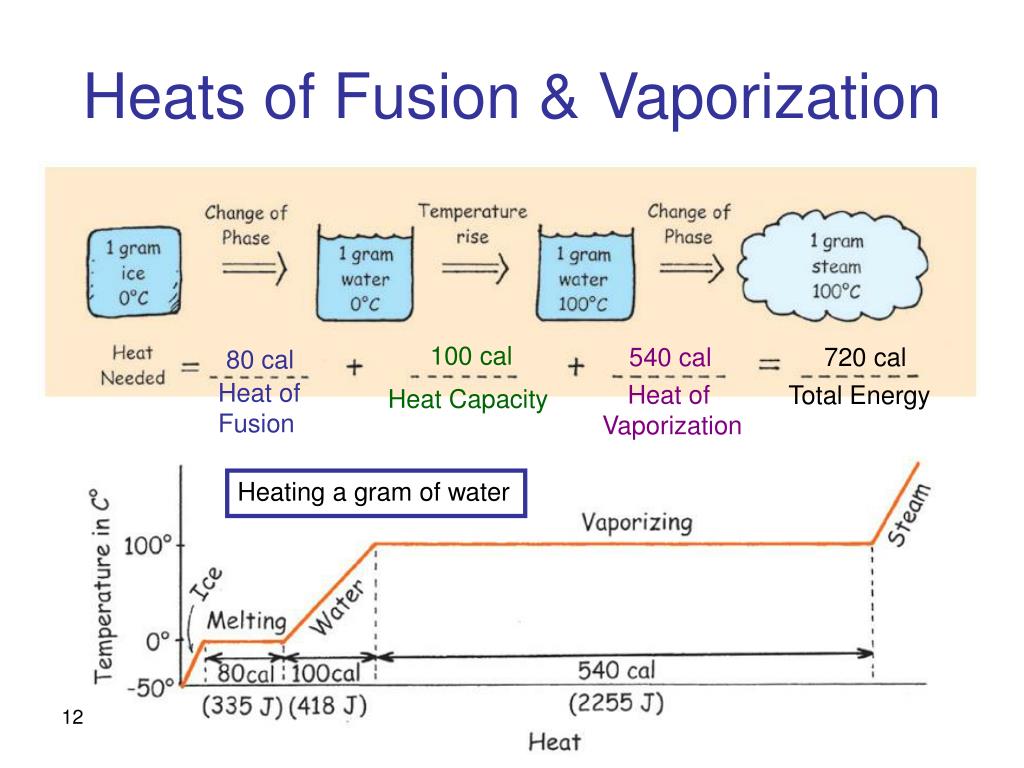
Heat And Phase Change Diagrams Chemistry Steps Phase changes transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat.if heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization) would. Phase changes, heats of fusion and vaporization, and phase diagra | channels for pearson . physics 20. heat and temperature calorimetry with temperature and phase changes. 4m. Table 11.3 latent heats of fusion and vaporization, along with melting and boiling points. let’s consider the example of adding heat to ice to examine its transitions through all three phases—solid to liquid to gas. a phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Q=mc Δt (energy of a temperature change within a phase) q=n Δh transition (energy of a phase transition) it needs to be realized that if you add heat, you move to the right, and if you remove heat, you move to the left. figure 10.3.2 10.3. 2: cooling curve for water. note, increasing heat moves to the left, removing heat moves to the right.
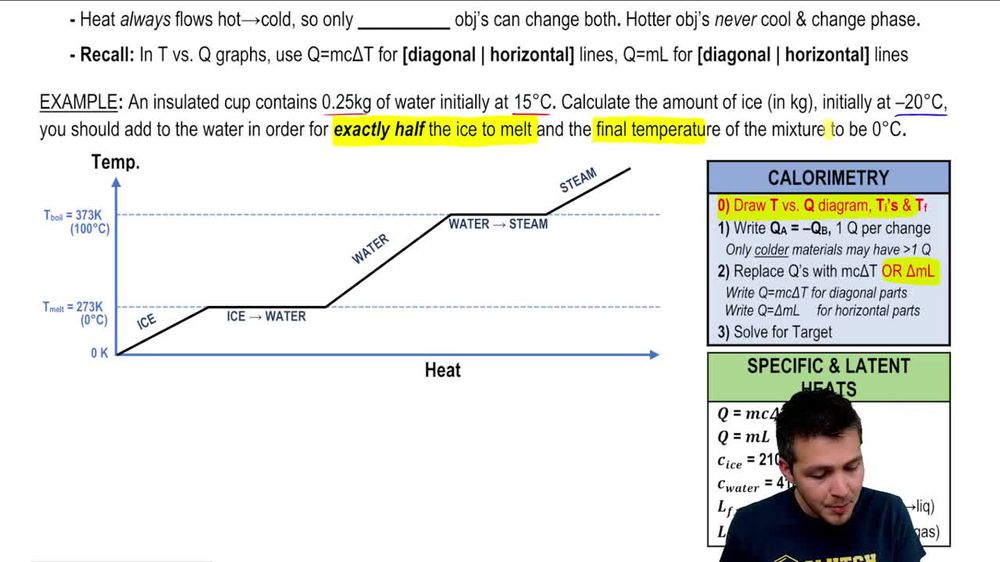
Phase Changes Heats Of Fusion And Vaporization And Phase Diagra Table 11.3 latent heats of fusion and vaporization, along with melting and boiling points. let’s consider the example of adding heat to ice to examine its transitions through all three phases—solid to liquid to gas. a phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Q=mc Δt (energy of a temperature change within a phase) q=n Δh transition (energy of a phase transition) it needs to be realized that if you add heat, you move to the right, and if you remove heat, you move to the left. figure 10.3.2 10.3. 2: cooling curve for water. note, increasing heat moves to the left, removing heat moves to the right. The energy per unit mass required to change a substance from the solid phase to the liquid phase, or released when the substance changes from liquid to solid, is known as the heat of fusion. the energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization. the strength of the. Phase transitions play an important theoretical and practical role in the study of heat flow. in melting (or “fusion”), a solid turns into a liquid; the opposite process is freezing. in evaporation, a liquid turns into a gas; the opposite process is condensation. a substance melts or freezes at a temperature called its melting point, and.
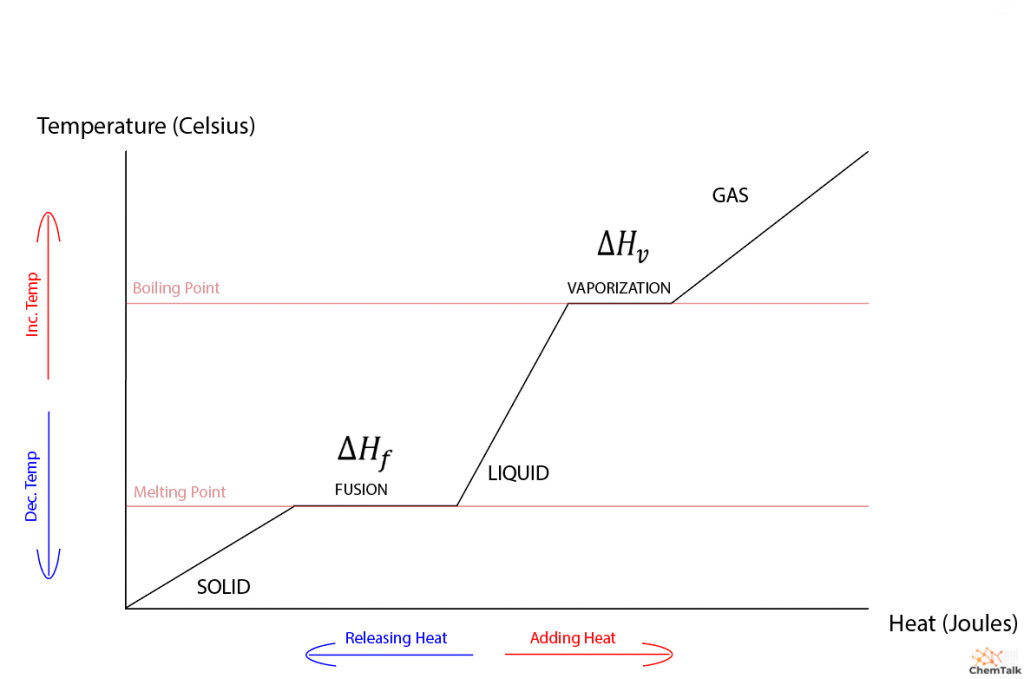
Heat Of Fusion Explained Chemtalk The energy per unit mass required to change a substance from the solid phase to the liquid phase, or released when the substance changes from liquid to solid, is known as the heat of fusion. the energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization. the strength of the. Phase transitions play an important theoretical and practical role in the study of heat flow. in melting (or “fusion”), a solid turns into a liquid; the opposite process is freezing. in evaporation, a liquid turns into a gas; the opposite process is condensation. a substance melts or freezes at a temperature called its melting point, and.
Ppt Chapter 17 Changes Of Phase Powerpoint Presentation Free

Comments are closed.