Ppt Heating Cooling Curve And Phase Diagrams Powerpoint Presentation

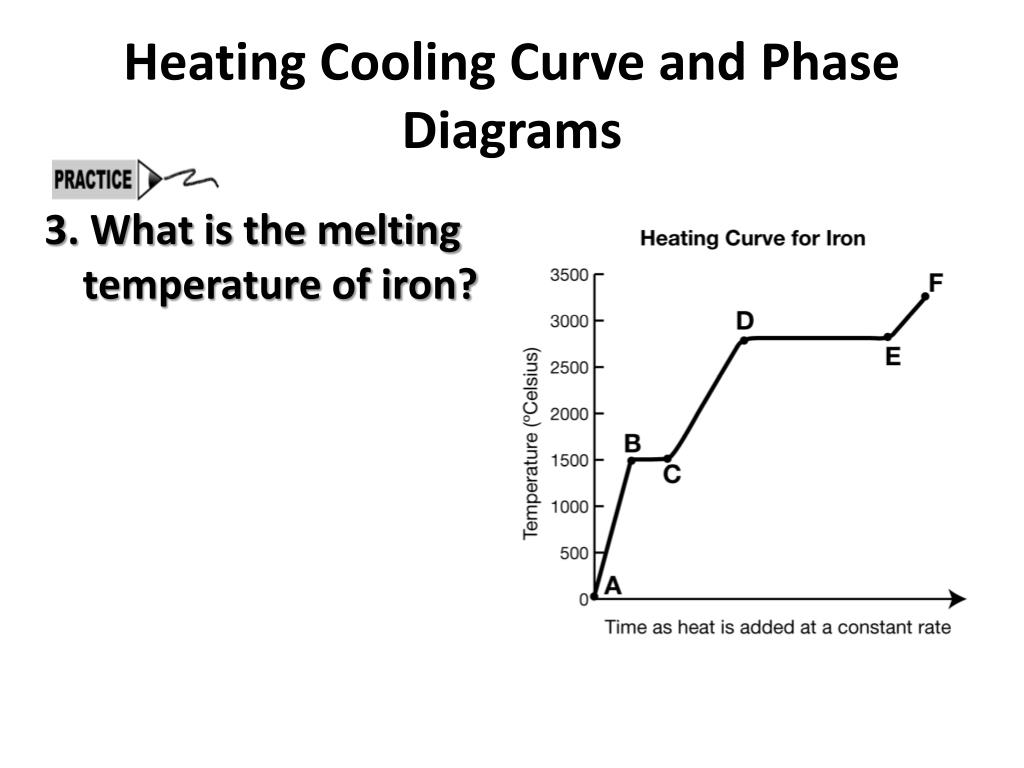
Ppt Heating Cooling Curve And Phase Diagrams Powerpoint Presentation Heating and cooling curves can show how temperature changes during these phase transitions. this document provides information about elements and compounds. it begins by defining pure substances as either elements or compounds. elements contain only one type of atom, while compounds contain two or more different types of atoms bonded together. Heating cooling curve and phase diagrams the heating curve at right shows the temperature change in a sample of iron as heat is added at a constant rate. the sample starts out as a solid and ends as a gas. • describe the phase change that occurred between points b and c on the graph. solution: between points b and c, the sample changed from.

Ppt Heating Cooling Curve And Phase Diagrams Powerpoint Presentation Heating and cooling curves for the sloped segments: q = m x cs x t. heating and cooling curves • calculate the h need to convert a 40.0 g block of ice at 22.0 c to steam at 115 c. the specific heats of ice, water, and steam are 2.03 j g k, 4.18 j g k, and 1.84 j g k respectively. for water, hfus = 6.01 kj mol and hvap = 40.67 kj mol. 3 heating cooling curve and phase diagrams. the heating curve at right shows the temperature change in a sample of iron as heat is added at a constant rate. the sample starts out as a solid and ends as a gas. describe the phase change that occurred between points b and c on the graph. solution: between points b and c, the sample changed from. The document summarizes key concepts about phase changes and energy changes, including: 1) phase changes can be exothermic or endothermic. 2) heating and cooling curves illustrate changes in kinetic and potential energy that occur during temperature and phase changes. 3) the specific heat capacity of a substance determines how much its. The document discusses heating and cooling curves, which are graphs of temperature over time that can be used to determine phase changes and melting boiling points of substances. heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. cooling curves are the opposite and show.

Ppt Heating Cooling Curve And Phase Diagrams Powerpoint Presentation The document summarizes key concepts about phase changes and energy changes, including: 1) phase changes can be exothermic or endothermic. 2) heating and cooling curves illustrate changes in kinetic and potential energy that occur during temperature and phase changes. 3) the specific heat capacity of a substance determines how much its. The document discusses heating and cooling curves, which are graphs of temperature over time that can be used to determine phase changes and melting boiling points of substances. heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. cooling curves are the opposite and show. Phase diagram and ttt diagram title: powerpoint author: cost last modified by: cost created date: 7 15 2003 12:36:03 pm document presentation format: | powerpoint ppt presentation | free to view figure 11.9: heating curve for water. 10 heating and cooling curves. heating and cooling curves are used to determine how energy influences phase changes during heating and cooling. all phase changes occur at constant temperature. therefore, average kinetic energy remains constant. during a phase change, the heat added (potential energy increases) or released (potential energy.
Ppt Heating Cooling Curve And Phase Diagrams Powerpoint Presentation Phase diagram and ttt diagram title: powerpoint author: cost last modified by: cost created date: 7 15 2003 12:36:03 pm document presentation format: | powerpoint ppt presentation | free to view figure 11.9: heating curve for water. 10 heating and cooling curves. heating and cooling curves are used to determine how energy influences phase changes during heating and cooling. all phase changes occur at constant temperature. therefore, average kinetic energy remains constant. during a phase change, the heat added (potential energy increases) or released (potential energy.

Comments are closed.