Pulmonary Arterial Hypertension Rx Combo Approved
Single Tablet Combo Of Macitentan And Tadalafil Receives Us Fda By lindsey shapiro, phd march 25, 2024. the u.s. food and drug administration (fda) has approved once daily, fixed dose tablets containing a combination of macitentan and tadalafil — to be marketed under the brand name opsynvi — to treat adults with pulmonary arterial hypertension (pah). the approval marks the first single tablet treatment. An investigational fixed dose combination of macitentan and tadalafil in a single tablet (m t stct) led to improvements in blood flow and reductions in a biomarker of heart failure for people with pulmonary arterial hypertension (pah) who were treatment naïve or previously received monotherapies, according to final data from the a due clinical trial.

Goal Oriented Combo Therapy Can Improves Hrqol In Pah Patients Modern treatment algorithms for pulmonary arterial hypertension (pah) using multiparametric risk stratification have improved outcomes for patients with pah ().currently, treatment algorithms propose upfront triple combination therapy, including a parenteral prostacyclin, for high risk patients, citing observational studies (), and upfront dual oral combination therapy for the majority of low. The u.s. food and drug administration (fda) has approved the under the skin injection therapy sotatercept csrk — to be marketed under the brand name winrevair — to treat adults with pulmonary arterial hypertension (pah). the now approved treatment is expected to be available for dispensing by select u.s. specialty pharmacies by the end of. Fda approves combination therapy for pah. j&j’s opsynvi is single tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a pde5 inhibitor. it will be priced on parity with opsumit, which is also a j&j product to treat patients with pulmonary arterial hypertension. the fda has approved opsynvi to treat adult. The bottom line. there are many medications available to manage pulmonary arterial hypertension (pah). examples of oral options include sildenafil (revatio, liqrev), tadalafil (adcirca, alyq, tadliq), and macitentan (opsumit). some people need two oral medications to improve their symptoms. infused medications are an option for more severe pah.

Of Fda Approved Drugs For Pulmonary Arterial Hypertension Download Fda approves combination therapy for pah. j&j’s opsynvi is single tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a pde5 inhibitor. it will be priced on parity with opsumit, which is also a j&j product to treat patients with pulmonary arterial hypertension. the fda has approved opsynvi to treat adult. The bottom line. there are many medications available to manage pulmonary arterial hypertension (pah). examples of oral options include sildenafil (revatio, liqrev), tadalafil (adcirca, alyq, tadliq), and macitentan (opsumit). some people need two oral medications to improve their symptoms. infused medications are an option for more severe pah. Letairis (ambrisentan) in combination with tadalafil (adcirca) approved to treat pulmonary arterial hypertension by fda. (rxwiki news) together, two medications for pulmonary arterial hypertension (pah) may help patients even more than either pill alone. that's why the us food and drug administration (fda) recently approved ambrisentan (brand. Combination therapy for pah approved by fda. october 13, 2015 by dr. jeremy feldman. for the first time, the fda has approved the use of two medications as initial therapy for pulmonary arterial hypertension. in response to the recently published ambition study results, the fda has approved the use of letairis (ambrisentan) and adcirca.

Pulmonary Arterial Hypertension Specific Therapies Approved By The Us Letairis (ambrisentan) in combination with tadalafil (adcirca) approved to treat pulmonary arterial hypertension by fda. (rxwiki news) together, two medications for pulmonary arterial hypertension (pah) may help patients even more than either pill alone. that's why the us food and drug administration (fda) recently approved ambrisentan (brand. Combination therapy for pah approved by fda. october 13, 2015 by dr. jeremy feldman. for the first time, the fda has approved the use of two medications as initial therapy for pulmonary arterial hypertension. in response to the recently published ambition study results, the fda has approved the use of letairis (ambrisentan) and adcirca.
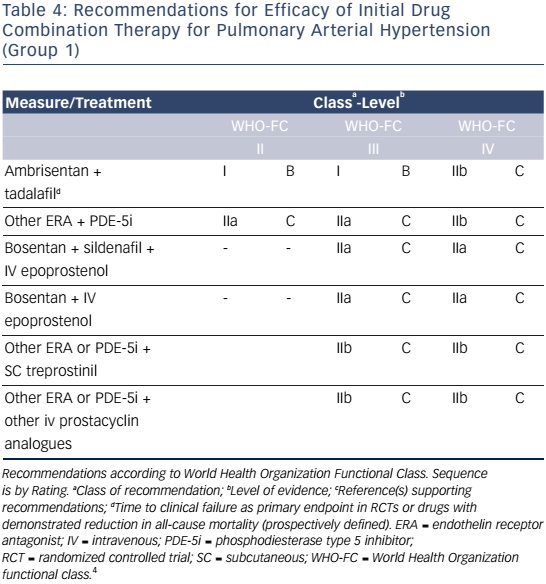
Pharmacologic Strategies For Management Of Pulmonary Arterial

Pdf Pharmacokinetic Interactions In Different Combinations Of

Comments are closed.