Q Calculate The Cfse For D7 D5 And D9 System For Low And High Spin Octahedral Complexes
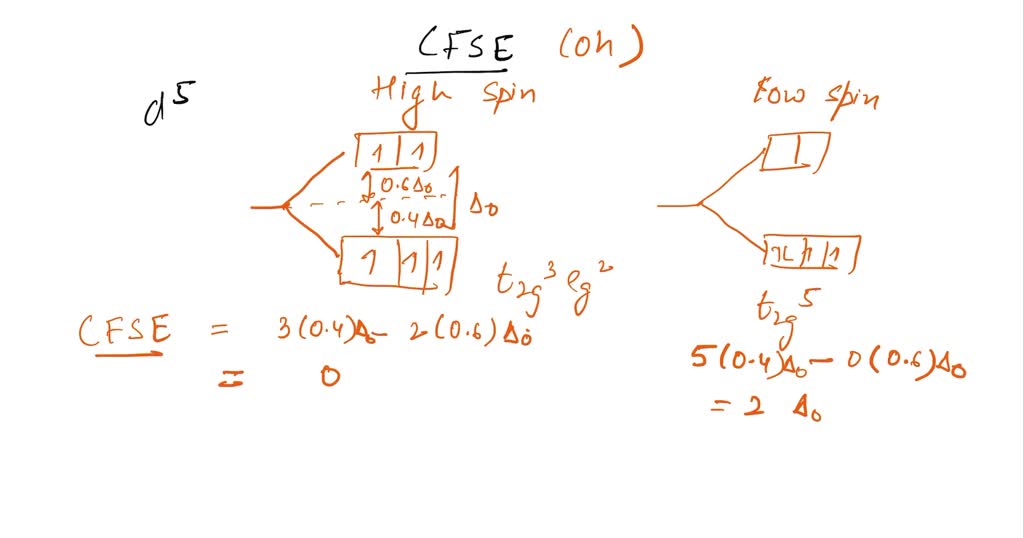
Q Calculate The Cfse For D7 D5 And D9 System For Low And High Spin Q .calculate the cfse for d7 d5 and d9 system for low and high spin octahedral complexes .? your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Notice that the spin pairing energy falls out in this case (and will when calculating the cfse of high spin complexes) since the number of paired electrons in the ligand field is the same as that in isotropic field of the free metal ion.
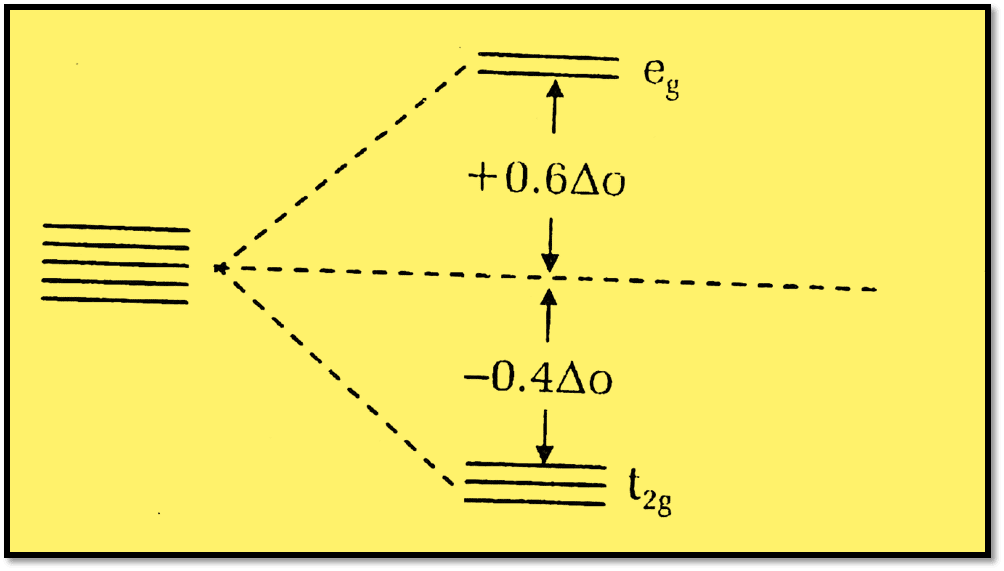
Crystal Field Stabilization Energy Cfse Definition Formula And 4 For each of these complexes we can calculate a crystal field stabilization energy, cfse, which is the energy difference between the complex in its ground state and in a hypothetical state in which all five d orbitals are at the energy barycenter. for ti 3 , there is one electron stabilized by 2 5 Δ o, so cfse = −(1)(25)(Δo) = −2 5 Δo c f. Calculate the crystal field stabilization energies (cfse or lfse) for d1,d2,d3,d4,d5, d6, d7, d8 ,d9 high spin octahedral and for low spin octahedral filed your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. The process is quite simple. to determine cfse we need to know that the diffidence of energy levels of two sets have been arbiter taken as 10 dq. . where t2g set is more stable then eg set octahedral and vice versa in tetrahedral complex. 0.4x 0.6y will help to calculate it. x indicates electrons in t2g and y in eg. * what is cfse* calculation of cfse in high & low spin complexes* some facts to be remembered while calculataing *for business enquires* [ contactstrongchem@.

Crystal Field Stabilization Energy Cfse Octahedral Complexes High The process is quite simple. to determine cfse we need to know that the diffidence of energy levels of two sets have been arbiter taken as 10 dq. . where t2g set is more stable then eg set octahedral and vice versa in tetrahedral complex. 0.4x 0.6y will help to calculate it. x indicates electrons in t2g and y in eg. * what is cfse* calculation of cfse in high & low spin complexes* some facts to be remembered while calculataing *for business enquires* [ contactstrongchem@. In octahedral system the amount of splitting is arbitrarily assigned to 10dq (oh). by using this calculator you can calculate crystal field stabilization energy for linear, trigonal planar, square planar , tetrahedral , trigonal bipyramid, square pyramidal, octahedral and pentagonal bipyramidal system (ligand field geometry). cite. Distribution of electrons in an octahedral complex d4 there are two possibilities for metal ions having d 4 d7 electronic configuration. depending on the nature of the ligands and the metal they could be high spin or low 2 u.e. spin complexes. 4 u.e. for the d4 system, cfse = for high spin, (3 × 0.4) – (1 × 0.6) = 0.6 Δ o and for low spin.
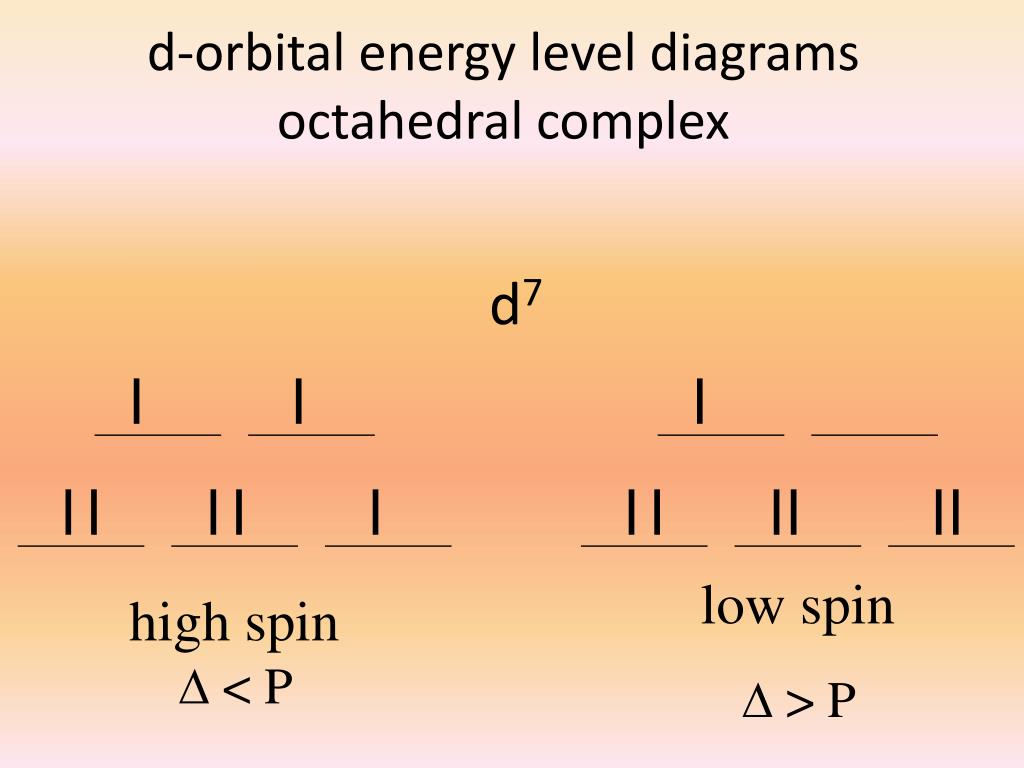
Ppt Crystal Field Theory Powerpoint Presentation Free Download Id In octahedral system the amount of splitting is arbitrarily assigned to 10dq (oh). by using this calculator you can calculate crystal field stabilization energy for linear, trigonal planar, square planar , tetrahedral , trigonal bipyramid, square pyramidal, octahedral and pentagonal bipyramidal system (ligand field geometry). cite. Distribution of electrons in an octahedral complex d4 there are two possibilities for metal ions having d 4 d7 electronic configuration. depending on the nature of the ligands and the metal they could be high spin or low 2 u.e. spin complexes. 4 u.e. for the d4 system, cfse = for high spin, (3 × 0.4) – (1 × 0.6) = 0.6 Δ o and for low spin.
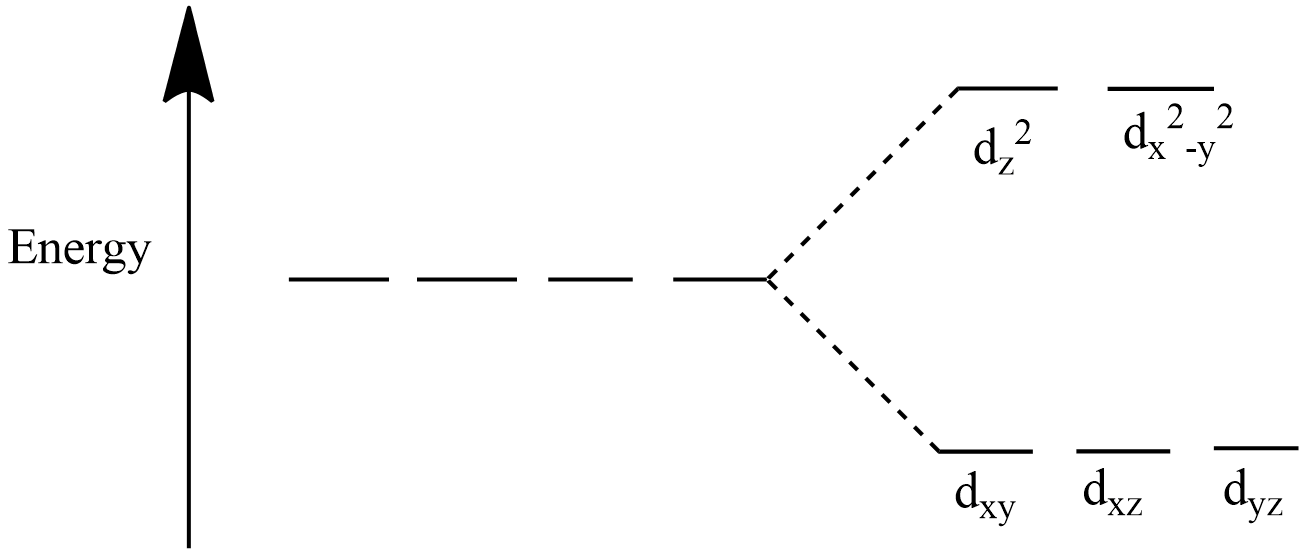
Energy Diagram For Octahedral Complexes

Solved 4 Low Spin Metals With A D6 Electronic Configurations In

Comments are closed.