Shapes Of Molecules Bond Angles As Chemistry Chemistry Education

Shapes Of Molecules Bond Angles As Chemistry Chemistry Education Figure 10.2.2): (cc by nc sa; anonymous) the two oxygens are double bonded to the sulfur. the oxygens have 2 lone pairs while sulfur had one lone pair. 3. there are two bonding pairs and one lone pair, so the structure is designated as ax 2 e. this designation has a total of three electron pairs, two x and one e. Explore molecule shapes by building molecules in 3d! how does molecule shape change with different numbers of bonds and electron pairs? find out by adding single, double or triple bonds and lone pairs to the central atom. then, compare the model to real molecules!.
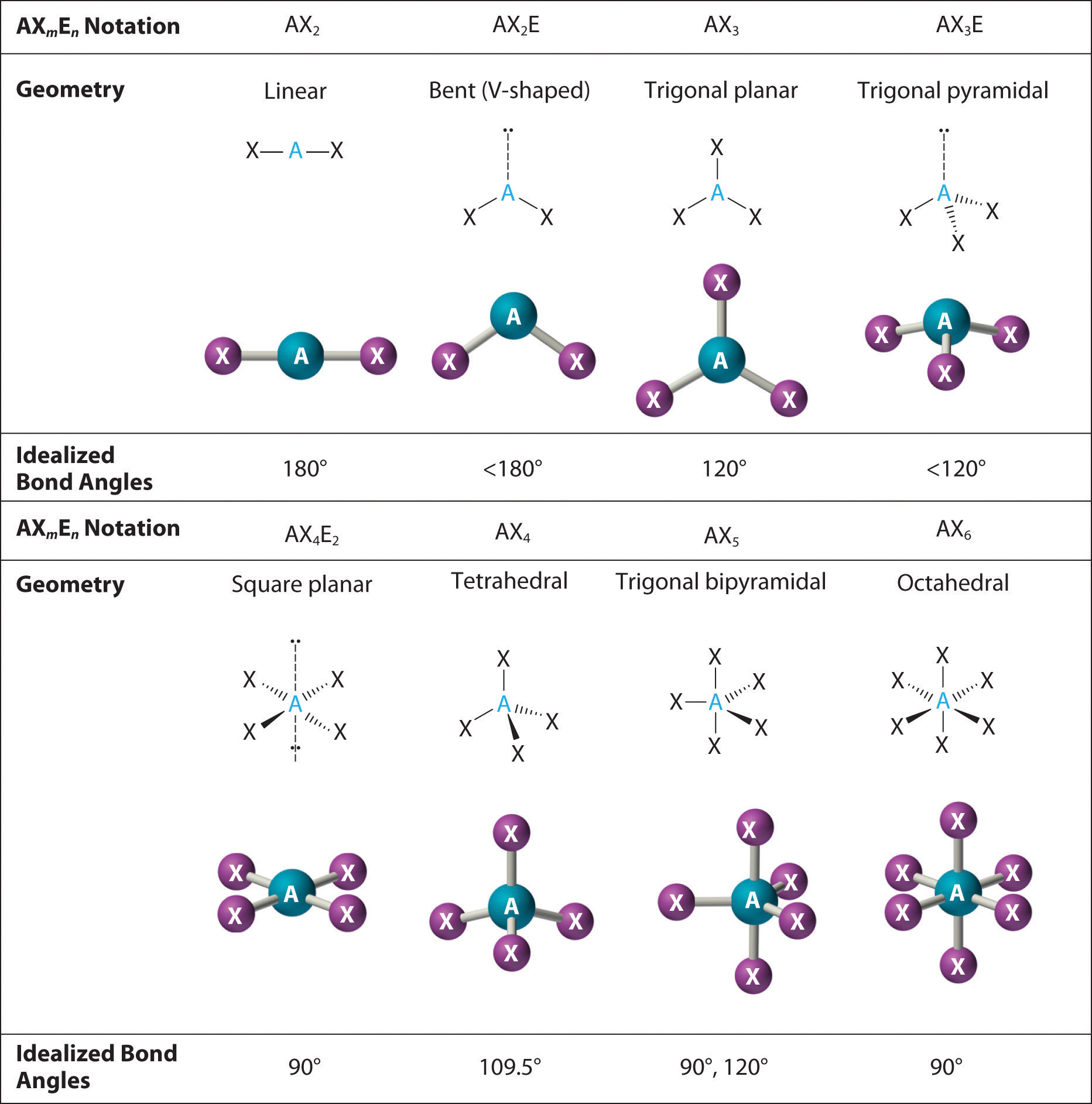
9 7 The Shapes Of Molecules Chemistry Libretexts Shapes of molecules. the valence shell electron pair repulsion theory (vsepr) predicts the shape and bond angles of molecules. electrons are negatively charged and will repel other electrons when close to each other. in a molecule, the bonding pair of electrons will repel other electrons around the central atom forcing the molecule to adopt a. The shape of any molecule or ion is a consequence of the number of electron pairs which repel each other as far as possible. lone pairs are more compact than bonding pairs. this means that lone pairs repel more than bonding pairs. this reduces the bond angles to a small extent. there are five basic shapes that you have to know, and all other. Worked example. vsepr & shapes of molecules. draw the shape of the following molecules: phosphorus (v) chloride. n (ch 3) 3. ccl 4. answer 1: phosphorus is in group 15, so has 5 valence electrons; cl is in group 17, so has 17 valence electrons. all 5 electrons are used to form covalent bonds with cl and there are no lone pairs. The lone pair will push the bond pairs outwards, reducing the bond angle to 107°. the resultant molecular shape is trigonal pyramidal. water (h 2 o) has two bond pairs and two lone pairs. the two lone pairs will repel the bond pairs further, thereby reducing the bond angle to 104.5°. the overall shape of the molecule is bent or angular. the.
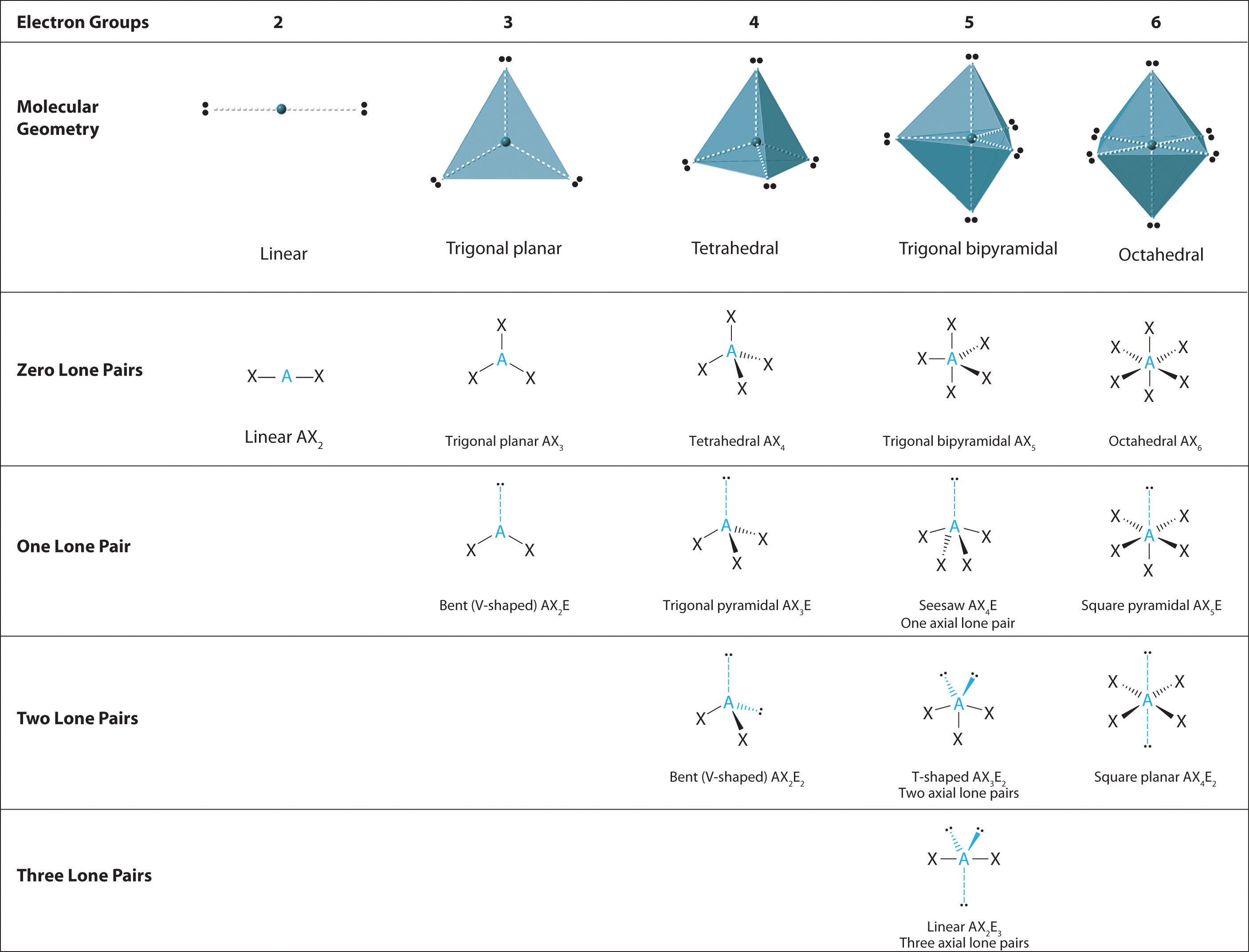
9 7 The Shapes Of Molecules Chemistry Libretexts Worked example. vsepr & shapes of molecules. draw the shape of the following molecules: phosphorus (v) chloride. n (ch 3) 3. ccl 4. answer 1: phosphorus is in group 15, so has 5 valence electrons; cl is in group 17, so has 17 valence electrons. all 5 electrons are used to form covalent bonds with cl and there are no lone pairs. The lone pair will push the bond pairs outwards, reducing the bond angle to 107°. the resultant molecular shape is trigonal pyramidal. water (h 2 o) has two bond pairs and two lone pairs. the two lone pairs will repel the bond pairs further, thereby reducing the bond angle to 104.5°. the overall shape of the molecule is bent or angular. the. Characteristics: three bonding pairs and one lone pair create this shape. bond angles: slightly less than 109.5°. bent molecules. examples: h₂o, so₂; characteristics: two bonding pairs and one or two lone pairs lead to a bent shape. bond angles: less than 120° for two lone pairs, about 120° for one lone pair. factors influencing. The effect of electron pair repulsion on bond angles. students should be able to explain the shapes of, and bond angles in, simple molecules and ions with up to six electron pairs (including lone pairs of electrons) surrounding the central atom. edexcel chemistry. topic 2: bonding and structure. topic 2a: bonding.

Comments are closed.