Solved Experiment 2 Chemical Kinetics Pre Lab Questions Chegg
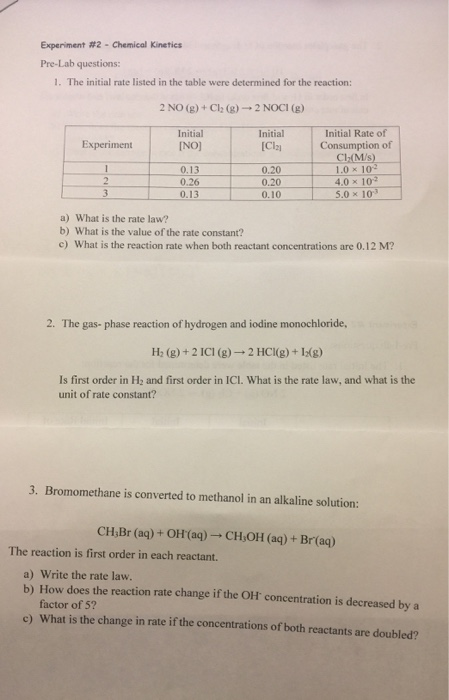
Solved Experiment 2 Chemical Kinetics Pre Lab Questions Chegg Chemistry questions and answers. experiment #2 chemical kinetics pre lab questions: 1. the initial rate listed in the table were determined for the reaction: 2 no (8) cl (8) 2 noci (8) experiment initial [no] initial [cla 0.13 0.26 0.13 initial rate of consumption of ch (m s) 1.0 x 10 4.0 x 10° 5.0 x 10 0.20 0.20 0.10 a) what is the. Solution. share share. 1. the indicator used in the experiment is starch. starch forms a complex with iodine. the complex is deep blue in colour. when potassium iodate reacts with sodium sulphide, iodine and sulphate salt is formed. the progress of reaction is checked by s …. view the full answer.

Solved Laboratory 2 Procedure Chemical Kinetics Chegg Chemistry questions and answers; pre lab: experiment 10; chemical kinetics 1. in an iodination of acetone reaction the following solutions make up the reaction mixture: 5 ml 4.0 m acetone 5 ml 0.0050 m 12 5 ml 1.0 m hci 10 ml h:0 a. what were the initial concentrations of acetone, 12 and h* ion in the reaction mixture?. Adjust your spectrophotometer to accurately read absorbances. 1. arrhenius equation. 2. on the graph x =. 3. while your cuvette is cooling in the ice bath, you will need to. 4. chemical kinetics: objective. Titration; titrations will be used to determine the concentration of hcl with respect to time. q2. which compound is added to slow down the chemical kinetics reaction the most? q2. propanone. q3. which paragraphs in the procedure will we be switching order to save an hour of time from the chemical kinetics experiment? q3. Study of kinetics. how fast a reaction will occur (rate of reaction) method 1. delta p delta t = mol min. method 2. rate = delta r delta t. why do we multiply by 1? the rate must be positive.

Solved Experiment 2 Pre Lab Questions 20 Points Stoichio Chegg Titration; titrations will be used to determine the concentration of hcl with respect to time. q2. which compound is added to slow down the chemical kinetics reaction the most? q2. propanone. q3. which paragraphs in the procedure will we be switching order to save an hour of time from the chemical kinetics experiment? q3. Study of kinetics. how fast a reaction will occur (rate of reaction) method 1. delta p delta t = mol min. method 2. rate = delta r delta t. why do we multiply by 1? the rate must be positive. Chem 102 experiment # chemical kinetics. pre lab activities: after reading the lab, complete items a, b, c, and d (title, purpose, chemicals and equipment, and summary of procedure) as described on page 10 of exp. 1 on an 8 1 2 x 11 sheet of paper. lab activities: go over the sample calculations with your lab instructor. 2 were varied. experiment [no] [h 2] initial rate in m s 1 0.15 m 0.35 0.56 2 0.24 m 0.35 1.43 3 0.24 m 0.48 1.96 to see the effect of changing the concentration of no, find two experiments where only the concentration of no is changing – experiment 1 and experiment 2 in this example. the rate laws for these experiments would be rate.

Solved Name Chem 132 Kinetics Week Pre Lab Problems 1 For Chegg Chem 102 experiment # chemical kinetics. pre lab activities: after reading the lab, complete items a, b, c, and d (title, purpose, chemicals and equipment, and summary of procedure) as described on page 10 of exp. 1 on an 8 1 2 x 11 sheet of paper. lab activities: go over the sample calculations with your lab instructor. 2 were varied. experiment [no] [h 2] initial rate in m s 1 0.15 m 0.35 0.56 2 0.24 m 0.35 1.43 3 0.24 m 0.48 1.96 to see the effect of changing the concentration of no, find two experiments where only the concentration of no is changing – experiment 1 and experiment 2 in this example. the rate laws for these experiments would be rate.
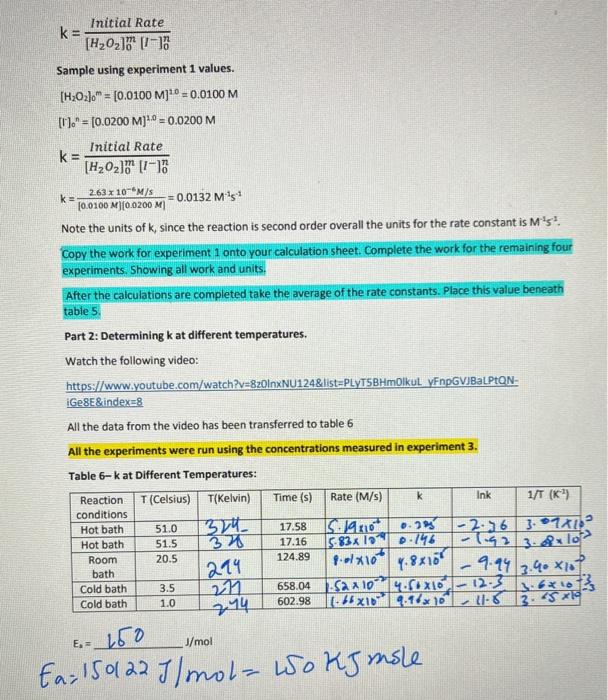
Solved Kinetics Lab Continued During Kinetics Part 1 You Chegg
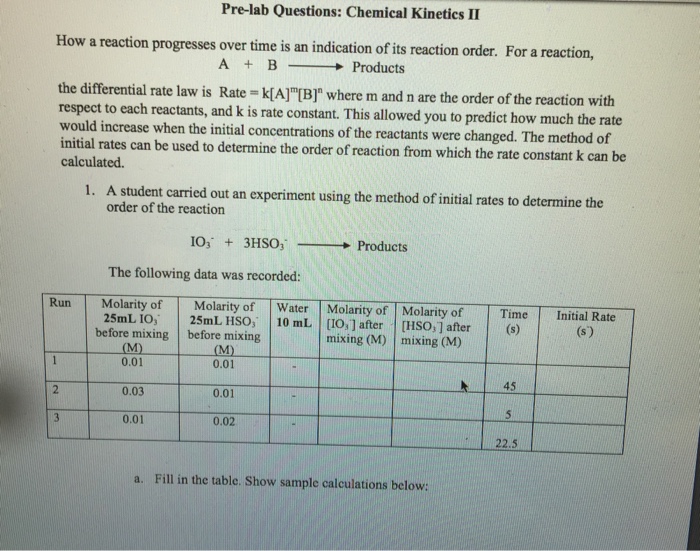
Solved Pre Lab Questions Chemical Kinetics Ii How A Chegg

Comments are closed.