Structure Of Water Molecule Chemistry Of Water Properties Of Water Composition Of Water
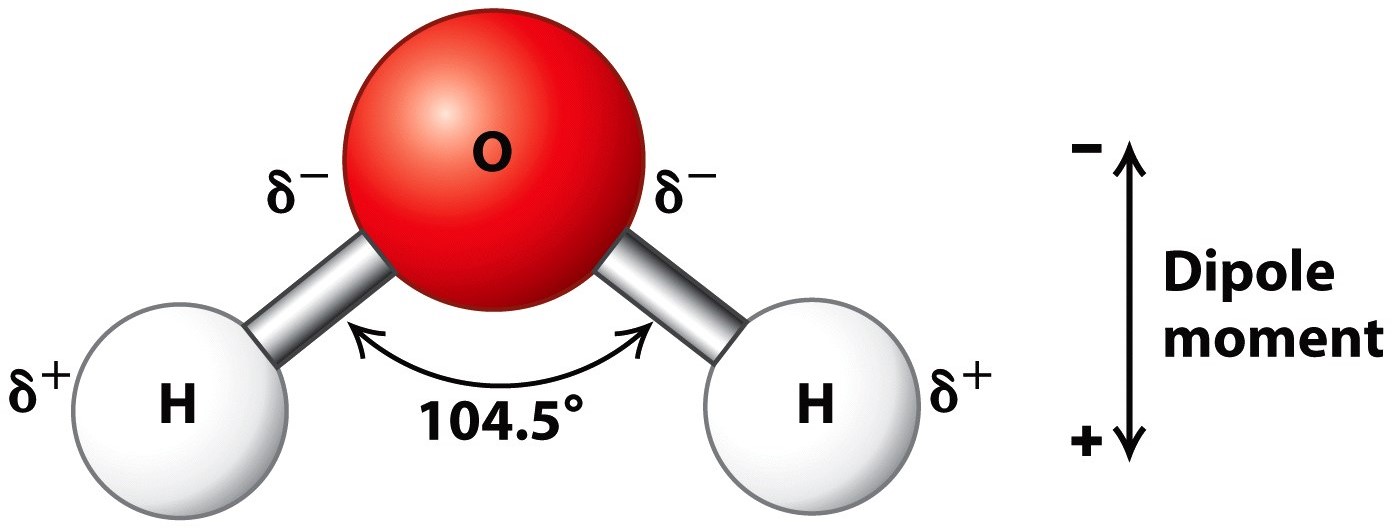
Water Structure And Properties Molecule Physical Properties A Level Structure of water. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (polar bonds). the oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. 13.5: the structure and properties of water. with 70% of our earth being ocean water and 65% of our bodies being water, it is hard to not be aware of how important it is in our lives. there are 3 different forms of water, or h 2 o: solid (ice), liquid (water), and gas (steam). because water seems so ubiquitous, many people are unaware of the.
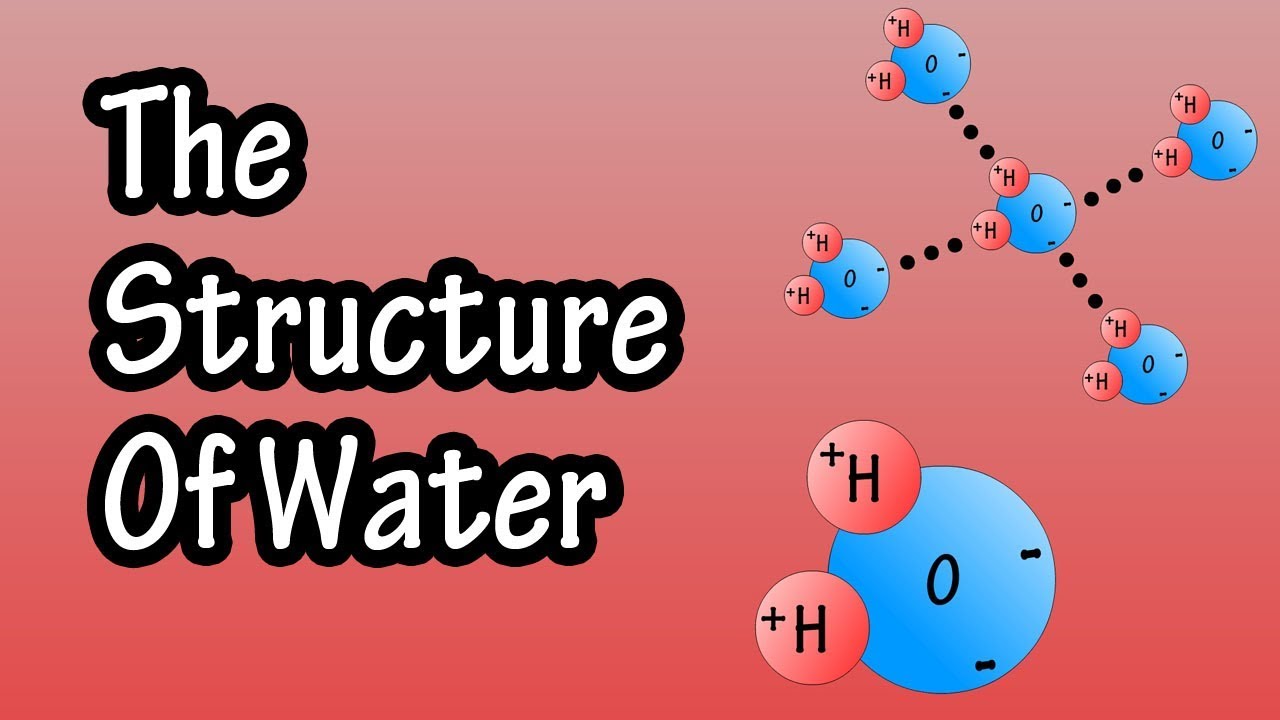
Structure Of Water Molecule Chemistry Of Water Properties Of Water In this video we discuss the structure of water. we cover how and why is water a solvent to other substances, and how the ability of water to act as a solve. Liquid water. water molecule a water molecule is made up of two hydrogen atoms and one oxygen atom. a single oxygen atom contains six electrons in its outer shell, which can hold a total of eight electrons. when two hydrogen atoms are bound to an oxygen atom, the outer electron shell of oxygen is filled. Selected physical properties of water are given in. table 1. to put these in context, comparison is made to the organic. solvents methanol and dimethyl ether, where one and two. of the hydrogen. Structure of water. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds). the oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms.

Water Definition Chemical Formula Structure Molecule Facts Selected physical properties of water are given in. table 1. to put these in context, comparison is made to the organic. solvents methanol and dimethyl ether, where one and two. of the hydrogen. Structure of water. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds). the oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. List of properties of water [1 4] structure. water consists of two atoms of hydrogen and one atom of oxygen. each of the two hydrogen atoms is covalently bonded with the oxygen atom, forming a tetrahedral shape. the water molecule is bent due to the repulsive effect of the lone pairs of electrons in oxygen. the h o h bond angle is 104.5 0. The molecule of water. a molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than h 2 o. in water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared.
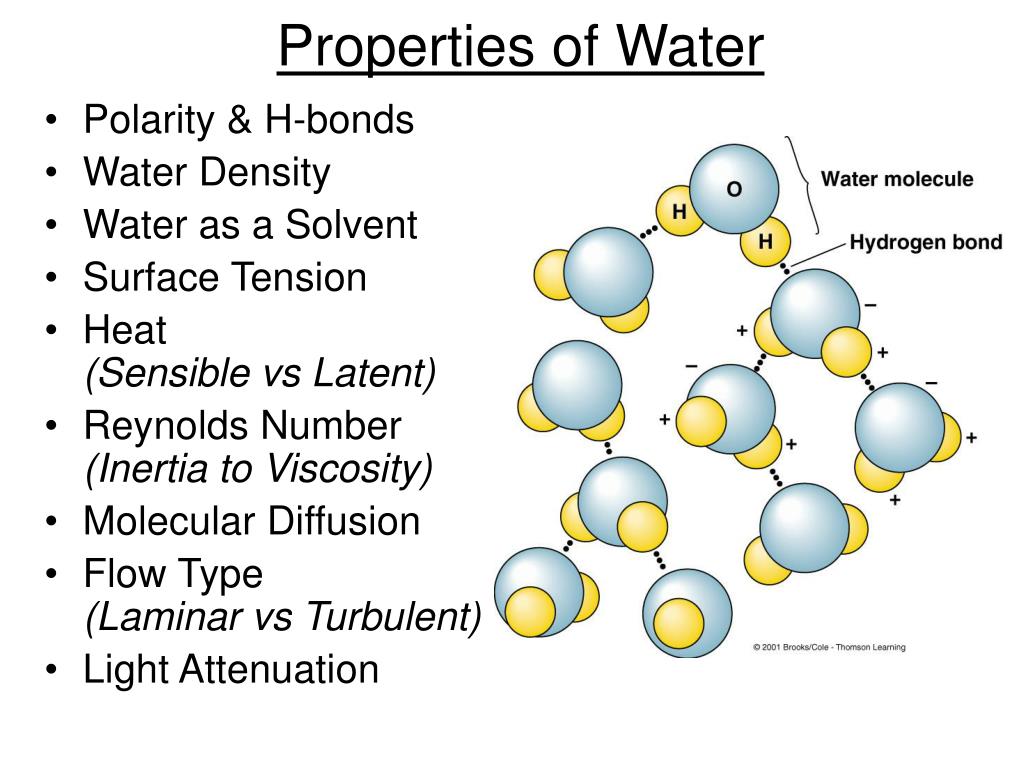
Ppt Properties Of Water Powerpoint Presentation Free Download Id List of properties of water [1 4] structure. water consists of two atoms of hydrogen and one atom of oxygen. each of the two hydrogen atoms is covalently bonded with the oxygen atom, forming a tetrahedral shape. the water molecule is bent due to the repulsive effect of the lone pairs of electrons in oxygen. the h o h bond angle is 104.5 0. The molecule of water. a molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than h 2 o. in water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared.

The Structure And Properties Of Water Introduction To Chemistry

Properties Of Water

Comments are closed.