The Atom Ppt Download
Ppt Structure Of Atom Powerpoint Presentation Free Download Id 6916836 Swbat determine the number of pen (protons, electrons and neutrons) in a neutral atom. 4 review what is an atom? • atom: the smallest unit of matter that retains the identity of the substance • first proposed by democratus 5 6 atomic structure • atoms are composed of 2 regions: 1.) nucleus the center of the atom that contains the mass. This document provides an outline for a presentation on the history and structure of atoms. it begins with the early ideas of democritus and thomson, then describes the atomic models of rutherford, bohr, and others. the structure of the atom is explained, including the nucleus, protons, neutrons, and electrons.

Ppt The Atom Powerpoint Presentation Free Download Id 1985546 Molecules are groups of atoms bonded together. ions are atoms or groups of atoms with a positive or negative charge. the mole concept relates the mass of a substance to its number of particles or molecules. 1) an atom is made up of protons, neutrons, and electrons. protons and neutrons are located in the nucleus, while electrons orbit the nucleus. 1. atoms are the basic building blocks of matter and consist of a small, dense nucleus surrounded by electrons. 2. rutherford's gold foil experiment in 1911 showed that the atom has a small, dense nucleus containing positively charged protons and uncharged neutrons. 3. 33 2.6 electronic structure of atoms. electrons in an atom are grouped around the nucleus into shells. the farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. the first shell (closest to the nucleus) can hold two electrons. Atoms contain a nucleus surrounded by an electron cloud that consists of one or more energy levels. 4 inner structure of an atom. nucleus small, dense, positively charged center of the atom which contains most of the atom’s mass. 5 inner structure of an atom. the nucleus contains the following subatomic particles: protons positively.

Ppt The Atom Powerpoint Presentation Free Download Id 5056341 33 2.6 electronic structure of atoms. electrons in an atom are grouped around the nucleus into shells. the farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. the first shell (closest to the nucleus) can hold two electrons. Atoms contain a nucleus surrounded by an electron cloud that consists of one or more energy levels. 4 inner structure of an atom. nucleus small, dense, positively charged center of the atom which contains most of the atom’s mass. 5 inner structure of an atom. the nucleus contains the following subatomic particles: protons positively. Dalton’s postulates 460 – 370 bc 1808 today 1. all elements are made of tiny indivisible particles called atoms. democritus atomism 2. all atoms of the same element are the same, but different from atoms of every other element. over 2,000 years later john dalton comes up with the first “modern” atomic theory. Structure of the atom.ppt free download as powerpoint presentation (.ppt), pdf file (.pdf), text file (.txt) or view presentation slides online. this document provides an overview of atomic structure and the particle theory of matter. it discusses the historical development of atomic models from dalton to bohr.
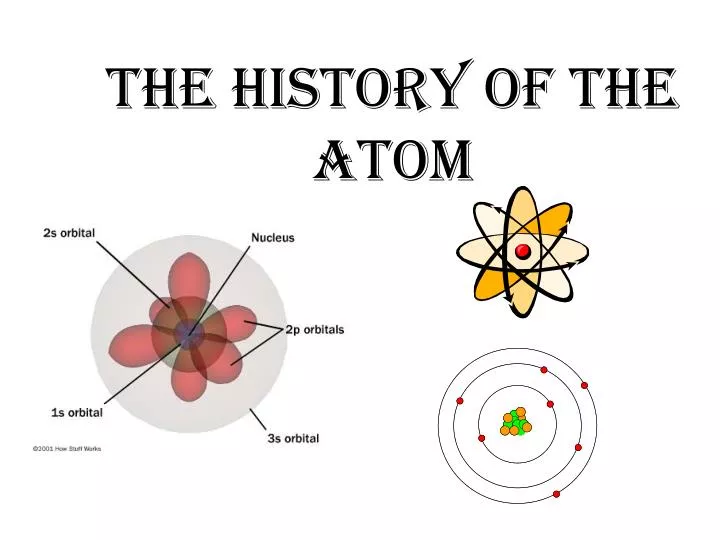
Ppt The History Of The Atom Powerpoint Presentation Free Download Dalton’s postulates 460 – 370 bc 1808 today 1. all elements are made of tiny indivisible particles called atoms. democritus atomism 2. all atoms of the same element are the same, but different from atoms of every other element. over 2,000 years later john dalton comes up with the first “modern” atomic theory. Structure of the atom.ppt free download as powerpoint presentation (.ppt), pdf file (.pdf), text file (.txt) or view presentation slides online. this document provides an overview of atomic structure and the particle theory of matter. it discusses the historical development of atomic models from dalton to bohr.

Comments are closed.