The Haber Bosch Process Nitrogen Fixing The World
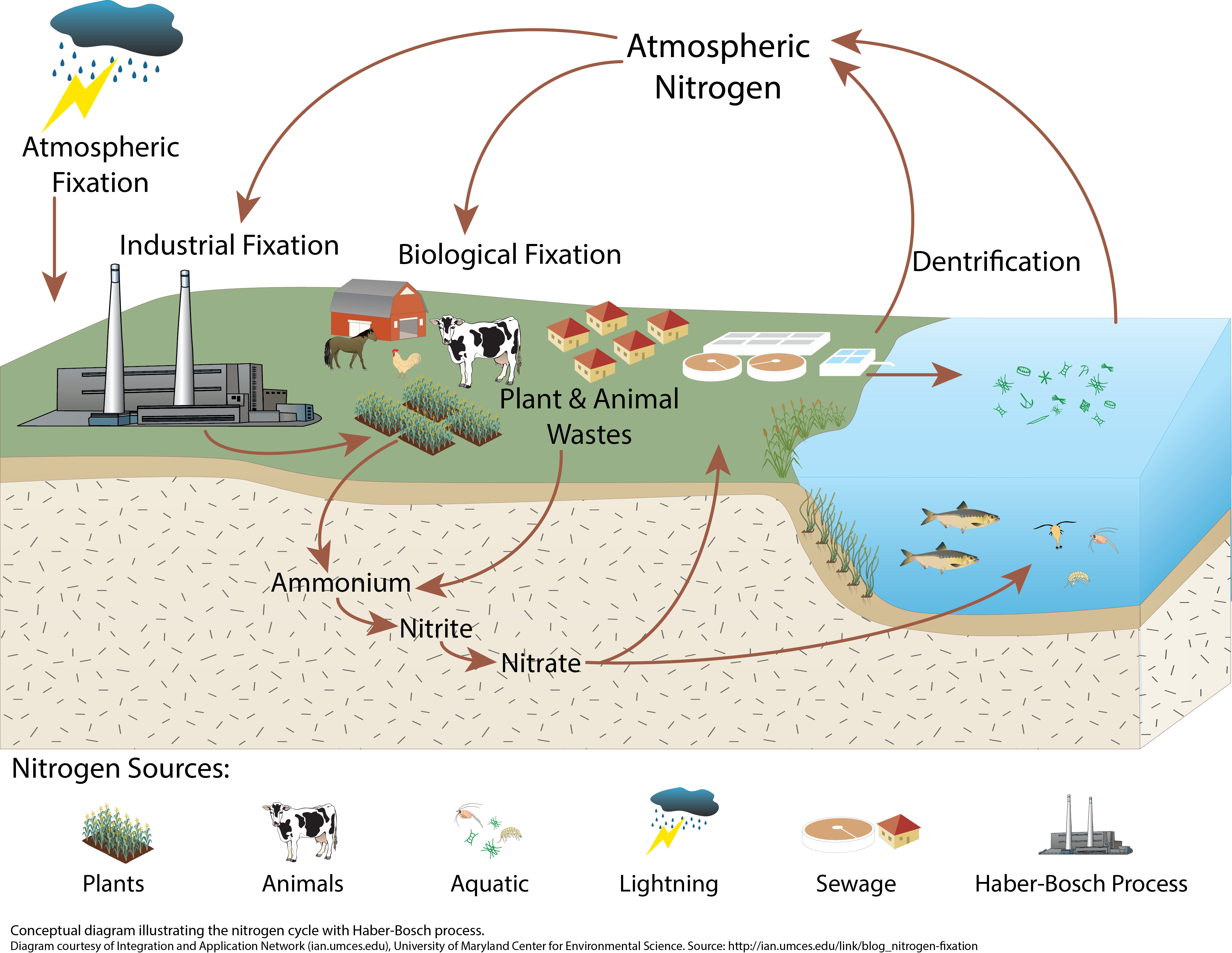
The Nitrogen Cycle With Haber Bosch Process Media Library A hundred years ago two german chemists, fritz haber and carl bosch, devised a way to transform nitrogen in the air into fertiliser, using what became known as the haber bosch process. but haber's. The haber process, [1] also called the haber–bosch process, is the main industrial procedure for the production of ammonia. [2][3] it converts atmospheric nitrogen (n 2) to ammonia (nh 3) by a reaction with hydrogen (h 2) using a finely divided iron metal catalyst: this reaction is slightly favorable in terms of enthalpy, but is disfavored in.

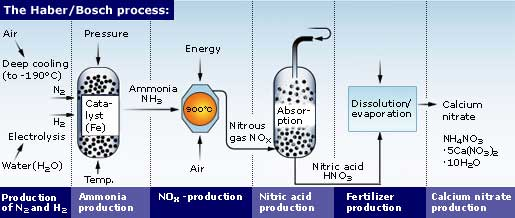
The Haber Bosch Process Nitrogen fixation. we spent most of chapter 22.1 discussing the biochemistry of nitrogenase which fixes the stable molecule n 2 to form nh 3 nh 4 . it's a very complicated reaction conducted by symbiotic microbes (prokaryotes) that fix n 2 for plants. the world uses the haber bosch process to produce over 100 million metric tons of nitrogen. The haber bosch process is the most economical for the fixation of nitrogen and with modifications continues in use as one of the basic processes of the chemical industry in the world. see also nitrogen fixation . The haber–bosch process has significantly lower energy requirements and was therefore substantially cheaper, allowing it to form the basis of an alternative expanding supply of reactive nitrogen. The haber bosch process converts atmospheric nitrogen (n 2) to ammonia (nh 3) by combining it with hydrogen (h 2). the process combines a single nitrogen molecule with 3 hydrogen molecules to produce 2 molecules of ammonia. the chemical equation for the haber bosch process is. n 2 3h 2 ⇌ 2nh 3. the ⇌ arrow in the above equation implies.
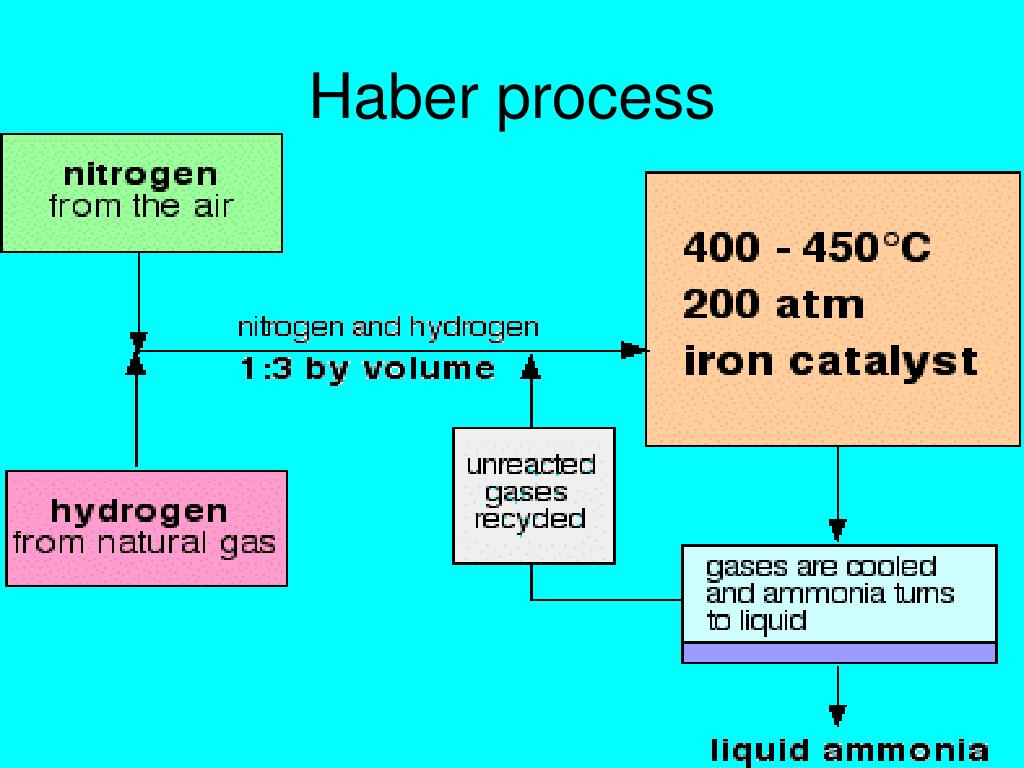
Ppt The Nitrogen Cycle Powerpoint Presentation Id 368207 The haber–bosch process has significantly lower energy requirements and was therefore substantially cheaper, allowing it to form the basis of an alternative expanding supply of reactive nitrogen. The haber bosch process converts atmospheric nitrogen (n 2) to ammonia (nh 3) by combining it with hydrogen (h 2). the process combines a single nitrogen molecule with 3 hydrogen molecules to produce 2 molecules of ammonia. the chemical equation for the haber bosch process is. n 2 3h 2 ⇌ 2nh 3. the ⇌ arrow in the above equation implies. The problem with nitrogen is that, while it is abundant in the atmosphere, its triple bonds make the nitrogen molecule incredibly stable and therefore hard to fix. haber was one of a group of chemists that also included walther nernst and henry le chatelier, which had decided to tackle the problem. without the haber bosch process we would only. The process of converting n 2 into biologically available nitrogen is called nitrogen fixation. n 2 gas is a very stable compound due to the strength of the triple bond between the nitrogen atoms.
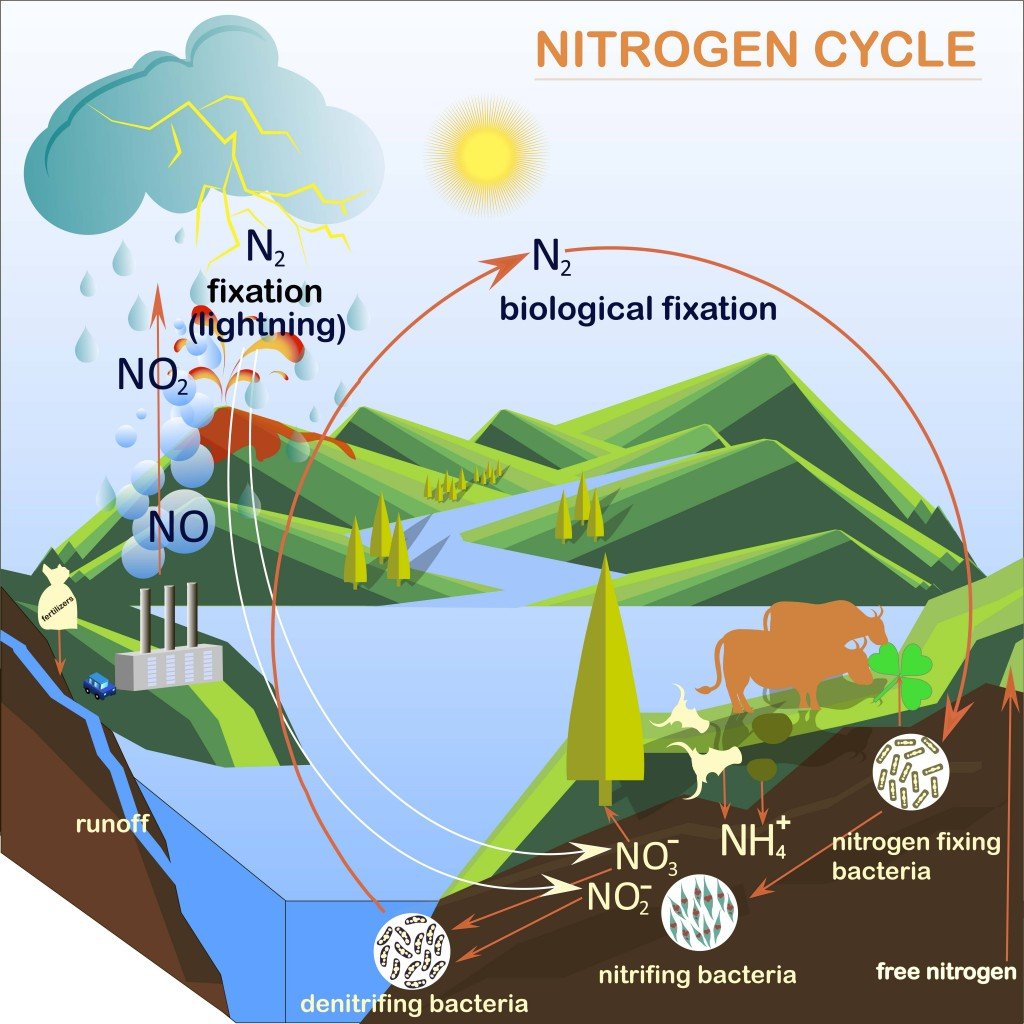
Haber Bosch Process Definition Equation Environmental Effects The problem with nitrogen is that, while it is abundant in the atmosphere, its triple bonds make the nitrogen molecule incredibly stable and therefore hard to fix. haber was one of a group of chemists that also included walther nernst and henry le chatelier, which had decided to tackle the problem. without the haber bosch process we would only. The process of converting n 2 into biologically available nitrogen is called nitrogen fixation. n 2 gas is a very stable compound due to the strength of the triple bond between the nitrogen atoms.
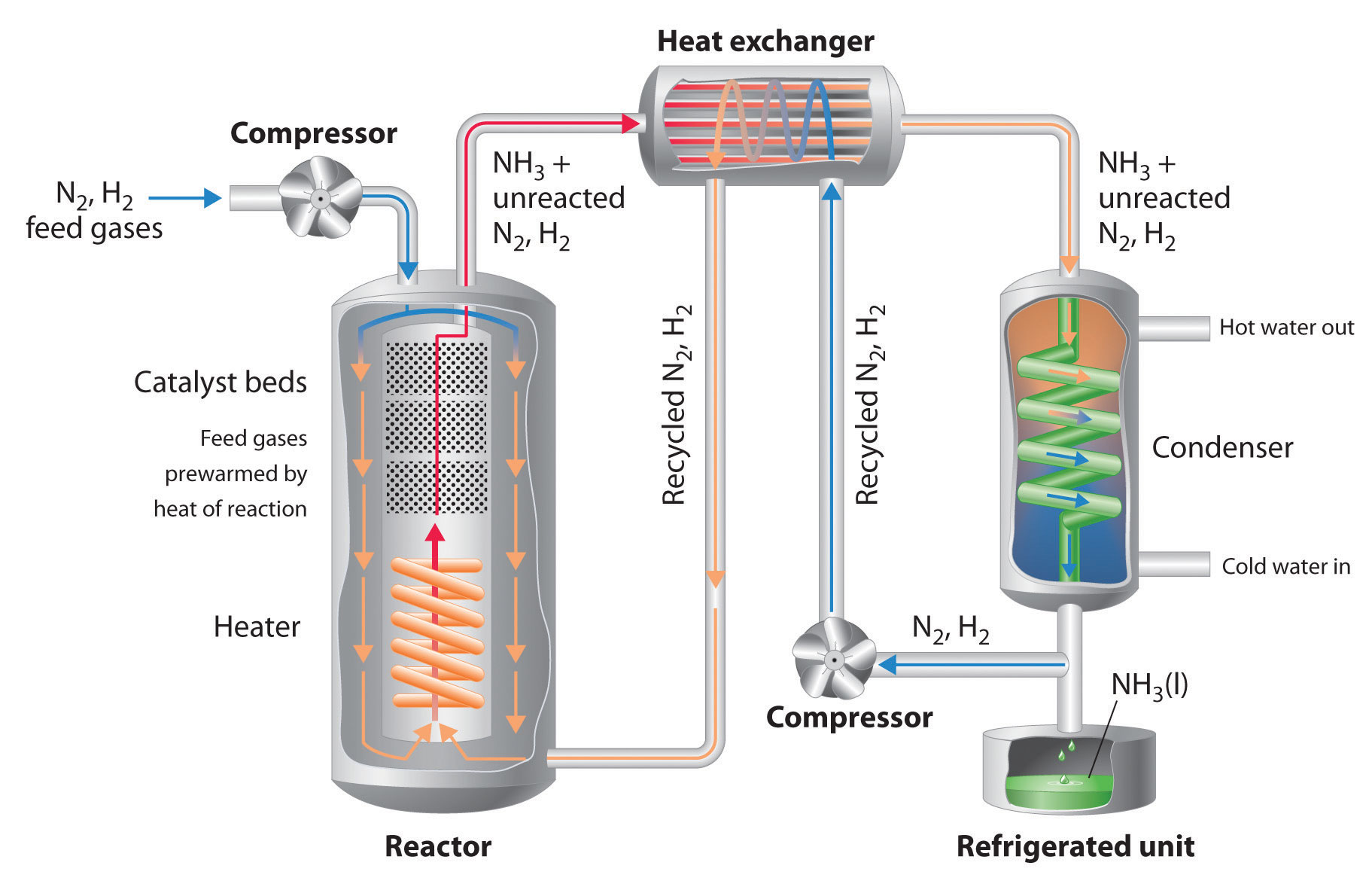
How Does The Haber Process Feed The World
Haber Bosch Process A Form Of Fertilize That Feeds The World Steemit

Comments are closed.