The Rate Constant For A First Order Reaction Becomes Six Times Class 12 Chemistry Doubtnut

The Rate Constant For A First Order Reaction Becomes Six Times When The The rate of a reaction becomes 4 times when temperature is raised from 293 k to 313 k. the activation energy for such reaction would be asked jul 14, 2019 in chemistry by kumari prachi ( 83.3k points). Step by step video, text & image solution for the rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction [r=8.314jk^( 1)mol^( 1)] by chemistry experts to help you in doubts & scoring excellent marks in class 12 exams.

The Rate Constant For A First Order Reaction Becomes Six Times When The T The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate activation energy for the reacti cbse exam, class 12. A first order reaction is 50% completed in 30 minutes at 300 k and in 10 minutes at 320 k, calculate the activation energy of the reaction (r = 8.314 jk mol − 1.) view solution the rate of a reaction triples when temperature changes from 50 ∘ c to 100 ∘ c. calculate the energy of activation for such a reaction. Thus, the activation energy for the reaction is $4.17 \times {10^4}{\text{ j mo}}{{\text{l}}^{ 1}}$. note: the rate of any chemical reaction is inversely proportional to the activation energy. higher the activation energy, slower is the chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction. (gas reactant r = 8.314 j k − 1 m o l − 1).
What Is The Unit Of Rate Constant For First Order Reaction Thus, the activation energy for the reaction is $4.17 \times {10^4}{\text{ j mo}}{{\text{l}}^{ 1}}$. note: the rate of any chemical reaction is inversely proportional to the activation energy. higher the activation energy, slower is the chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction. (gas reactant r = 8.314 j k − 1 m o l − 1). The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction ( r = 8.314 jk 1 mol 1 ) q. the rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for. The rate constant of a first order reaction is 6 times when the temperature is increased from 350 k to 410 k. the energy of activation for the reaction is (i n k j m o l − 1). write it in 10x form. ( write your answer to nearest integer).
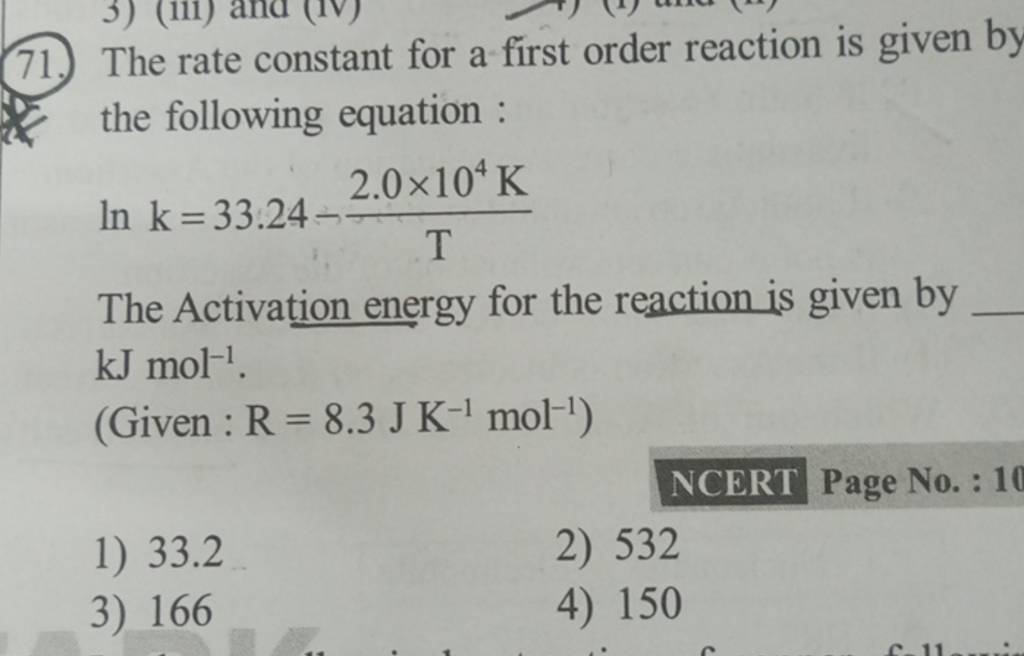
The Rate Constant For A First Order Reaction Is Given By The Following Eq The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction ( r = 8.314 jk 1 mol 1 ) q. the rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for. The rate constant of a first order reaction is 6 times when the temperature is increased from 350 k to 410 k. the energy of activation for the reaction is (i n k j m o l − 1). write it in 10x form. ( write your answer to nearest integer).

Comments are closed.